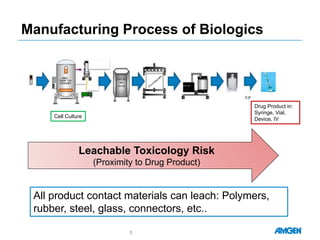
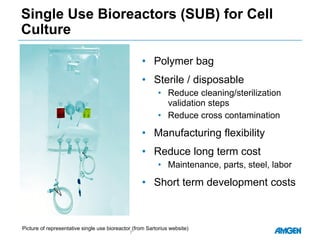
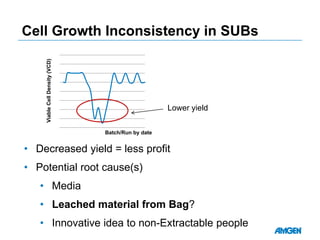
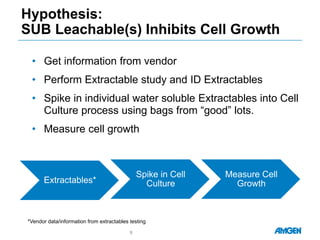
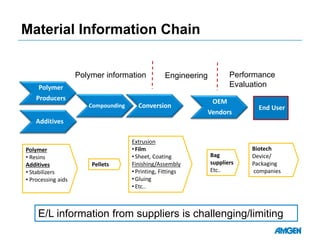
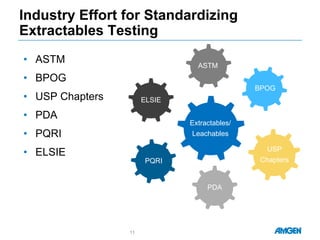
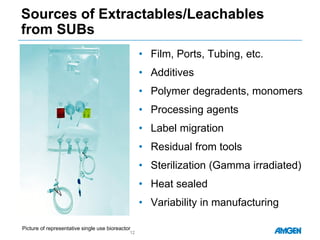
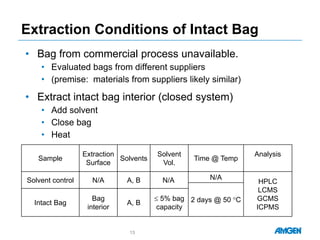
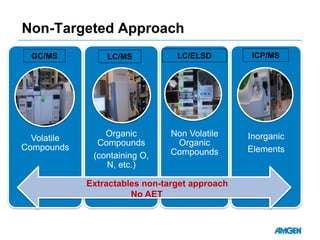
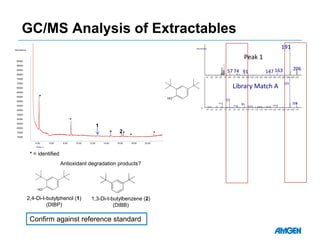
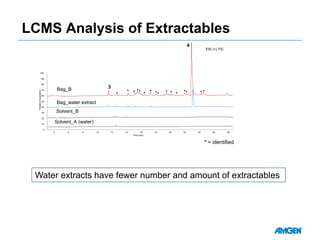
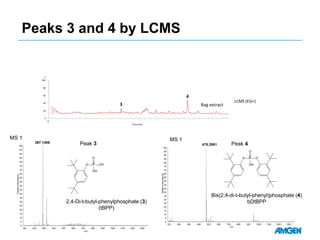
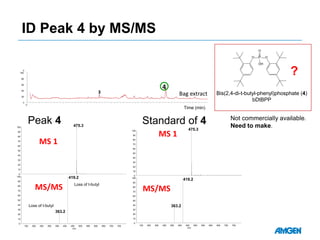
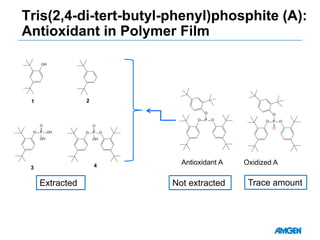
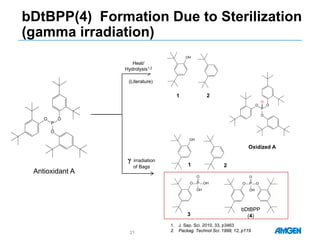
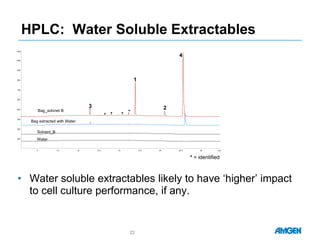
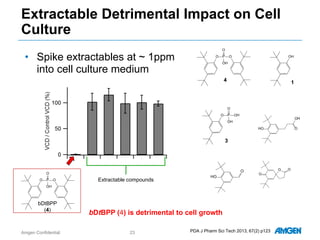
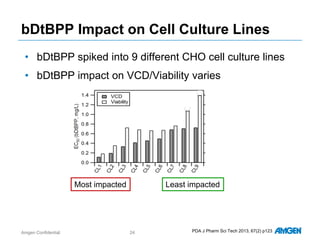
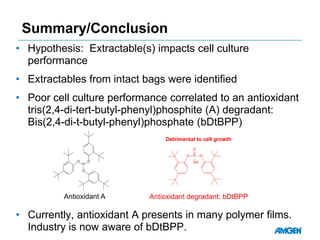
Extractables from single-use bioreactors were found to negatively impact cell culture performance. Testing identified various extractable molecules, including degradation products of the antioxidant tris(2,4-di-tert-butyl-phenyl)phosphite. Bis(2,4-di-t-butyl-phenyl)phosphate (bDtBPP) in particular was shown to inhibit cell growth. The findings suggest controlling raw materials and manufacturing processes to minimize extractable contaminants that harm cells.