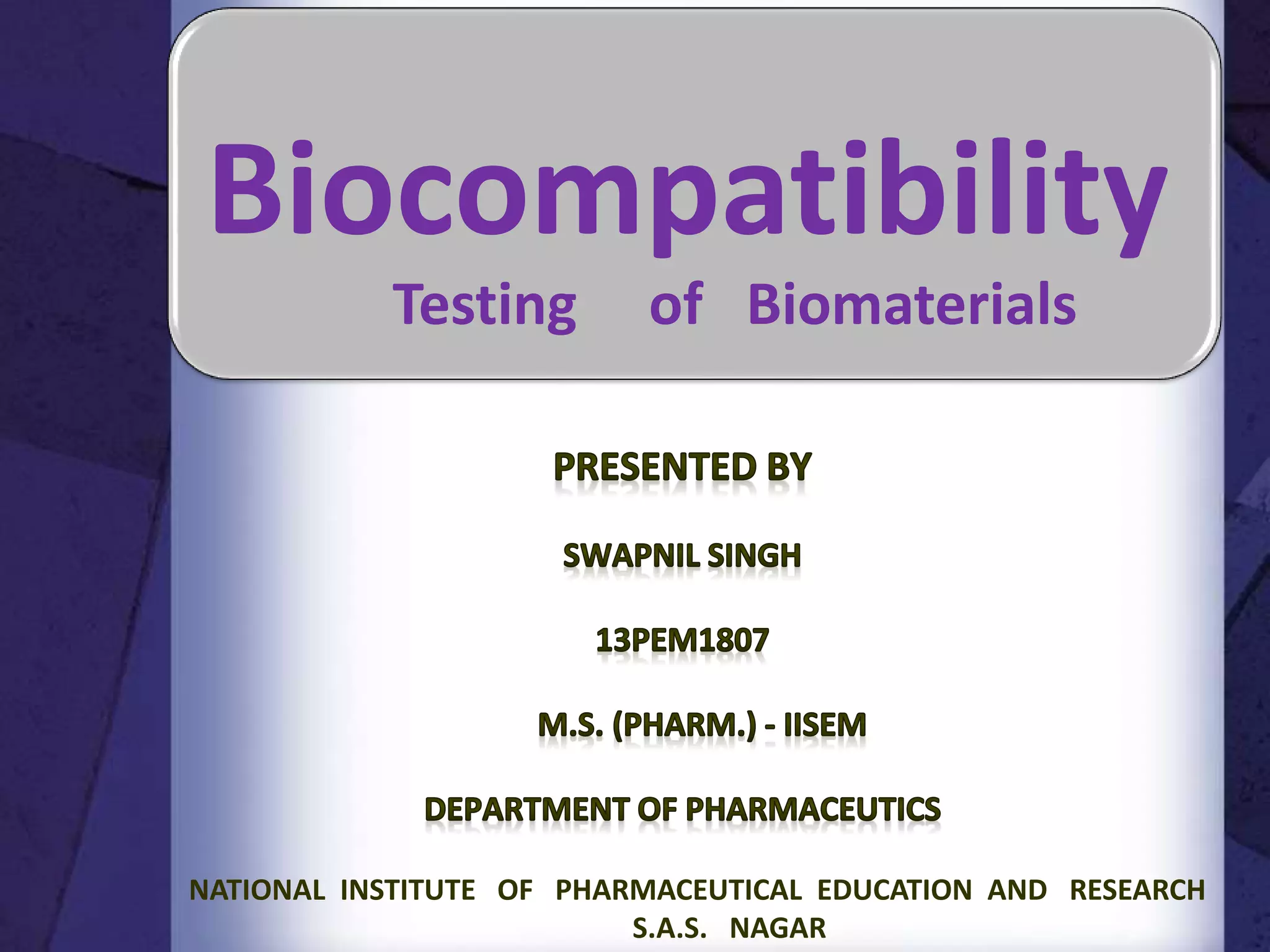
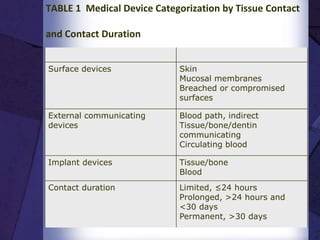
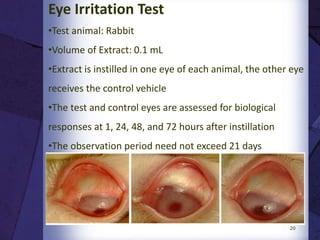
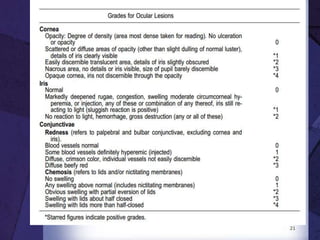
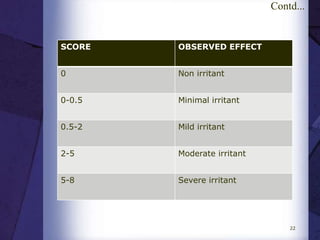
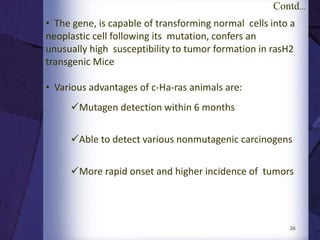
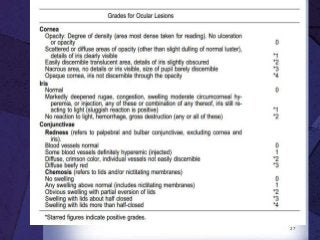
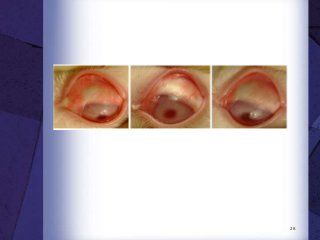
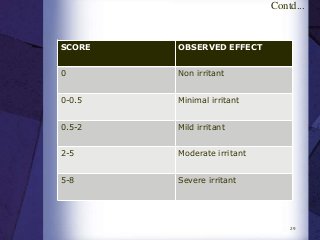
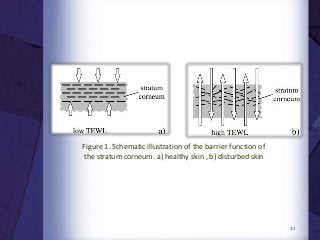
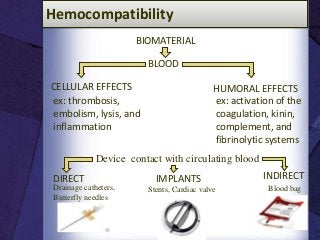
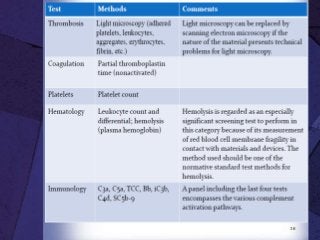
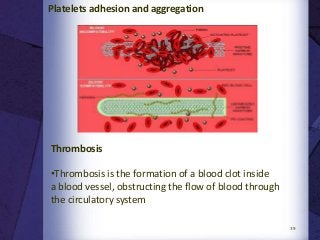
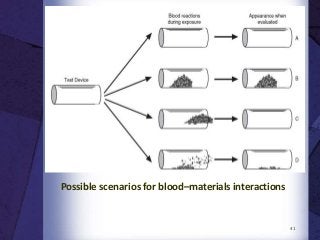
The document discusses biocompatibility, emphasizing the need for materials to function safely within the body without adverse reactions. It outlines various testing methodologies including in vitro and in vivo tests for evaluating biocompatibility, detailing their advantages, disadvantages, and specific tests per ISO standards. Key testing parameters also include acute and chronic toxicity, genotoxicity, and hemocompatibility, with protocols for testing and evaluation to ensure safety in medical devices.