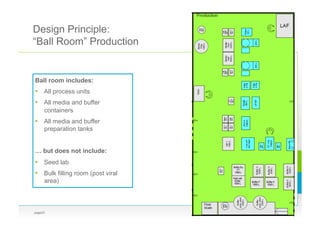
Berthold Boedeker from Bayer Pharma AG gave a presentation on recent innovations in biologics manufacturing, including the benefits and challenges of disposables, continuous processing, and new facility designs. He discussed how disposables can simplify processing but have limitations from vendor standardization issues. New facility concepts like the "ballroom plant" aim to allow parallel production of multiple products with lower segregation requirements through closed and continuous processing based on disposables. Continuous perfusion and downstream processing using disposables could allow for smaller, less expensive and more flexible facilities.




![Best-Selling Pharmaceutical Products
Page 5
[ € million ]
%
First Nine
Months 2015
First Nine
Months 2014
Change
Fx adj.
%
3rd Quarter
2015
Xarelto™ 1,163 1,602 +37.7 +37.1
Eylea™ 540 874 +61.9 +57.1
Kogenate™ 808 869 +7.5 +0.7
Mirena™ product family 594 742 +24.9 +9.8
Nexavar™ 571 661 +15.8 +6.1
Betaferon™/Betaseron™ 629 634 +0.8 -9.2
YAZ™/Yasmin™/Yasminelle™ 570 538 -5.6 -5.0
Adalat™ 435 481 +10.6 +0.8
Aspirin™ Cardio 356 393 +10.4 +2.3
Glucobay™ 310 381 +22.9 +4.1
Avalox™/Avelox™ 285 294 +3.2 -3.5
Stivarga™ 161 236 +46.6 +29.3
Xofigo™ 128 188 +46.9 +27.5
Total 6,861 8,189 +19.4 +12.0
Proportion of Pharmaceuticals sales 78% 80%
Fx & p adj. = currency- and portfolio-adjusted](https://image.slidesharecdn.com/bayer2016-160308122303/85/Boedeker-Bayer-BILS-2016-5-320.jpg)




















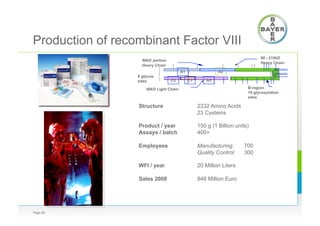
![Long Term Continuous Fermentation of
rec FVIII
Page 29
Time t [d]
Cellconcentration[106vc/mL]
Viability[%]
10
100
1
10
100
0 20 40 60 80 100 120 140
1
Cell concentration
Viability
production of unstable
protein
q/V = 10 /d
Dr. Konstantinov, Bayer Corp.,
Dechema 2002, Frankfurt](https://image.slidesharecdn.com/bayer2016-160308122303/85/Boedeker-Bayer-BILS-2016-29-320.jpg)