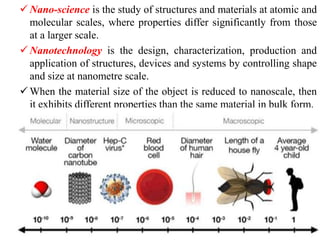
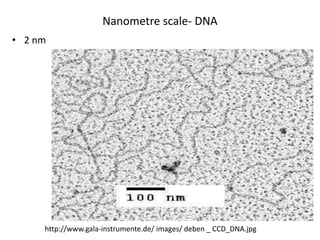
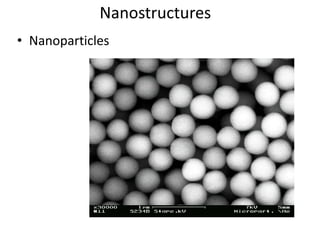
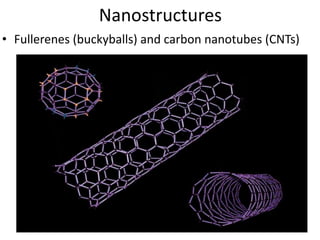
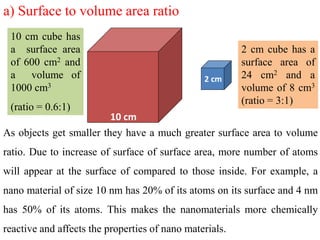
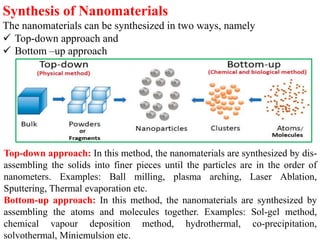
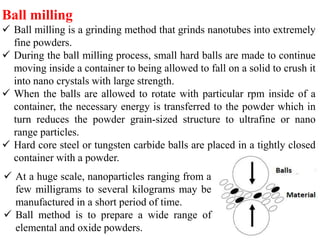
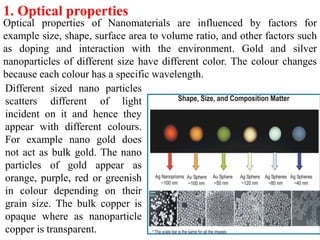
The document provides an overview of nanomaterials, covering their significance, properties, and synthesis methods, as well as their applications in various fields. It distinguishes nanomaterials into three categories based on dimensions and explains the impact of nanoscale on physical and chemical properties due to increased surface area and quantum effects. Key synthesis techniques discussed include top-down and bottom-up approaches, while highlighting the influence of size on properties like optical, thermal, magnetic, and chemical behavior.