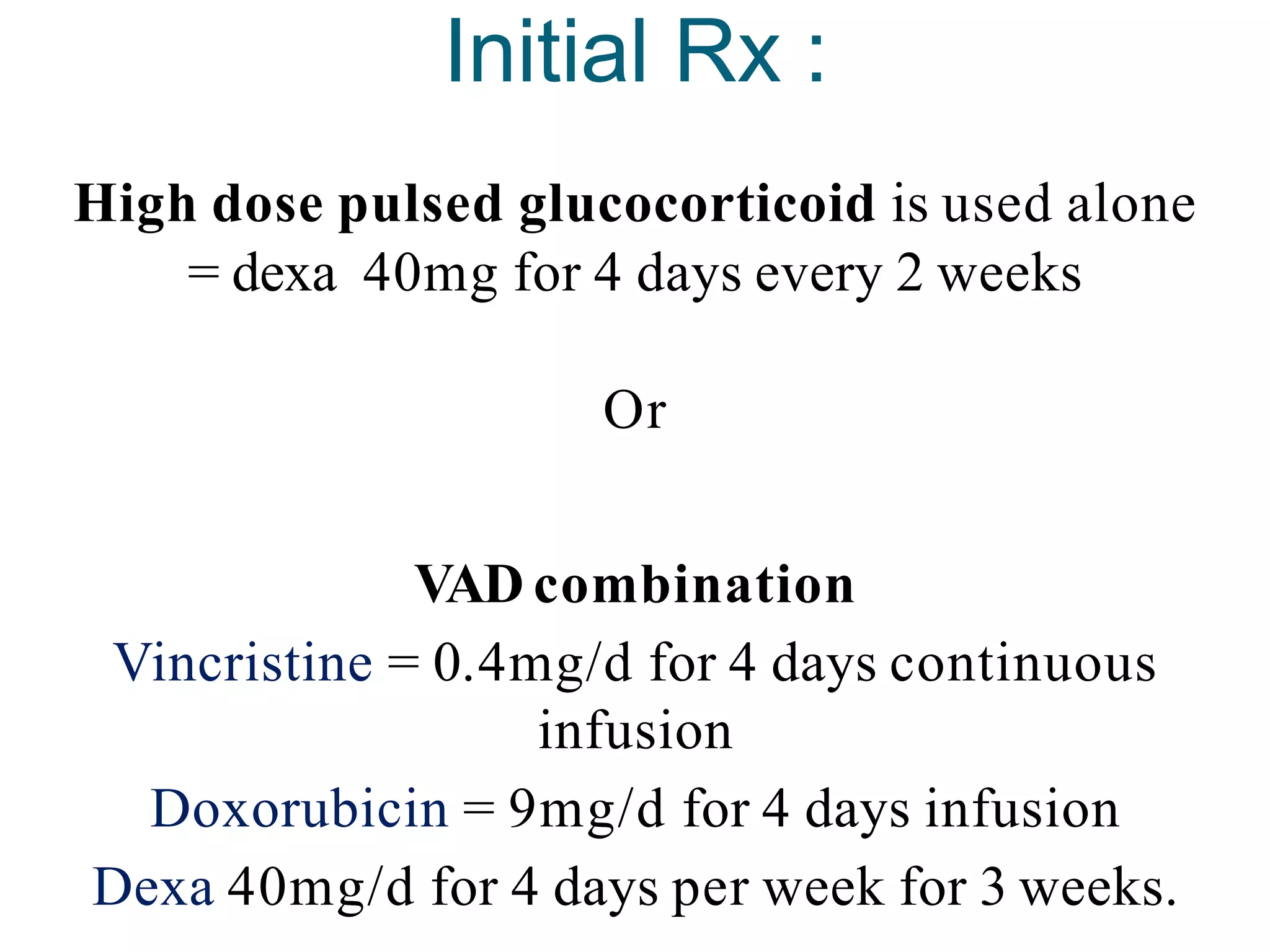
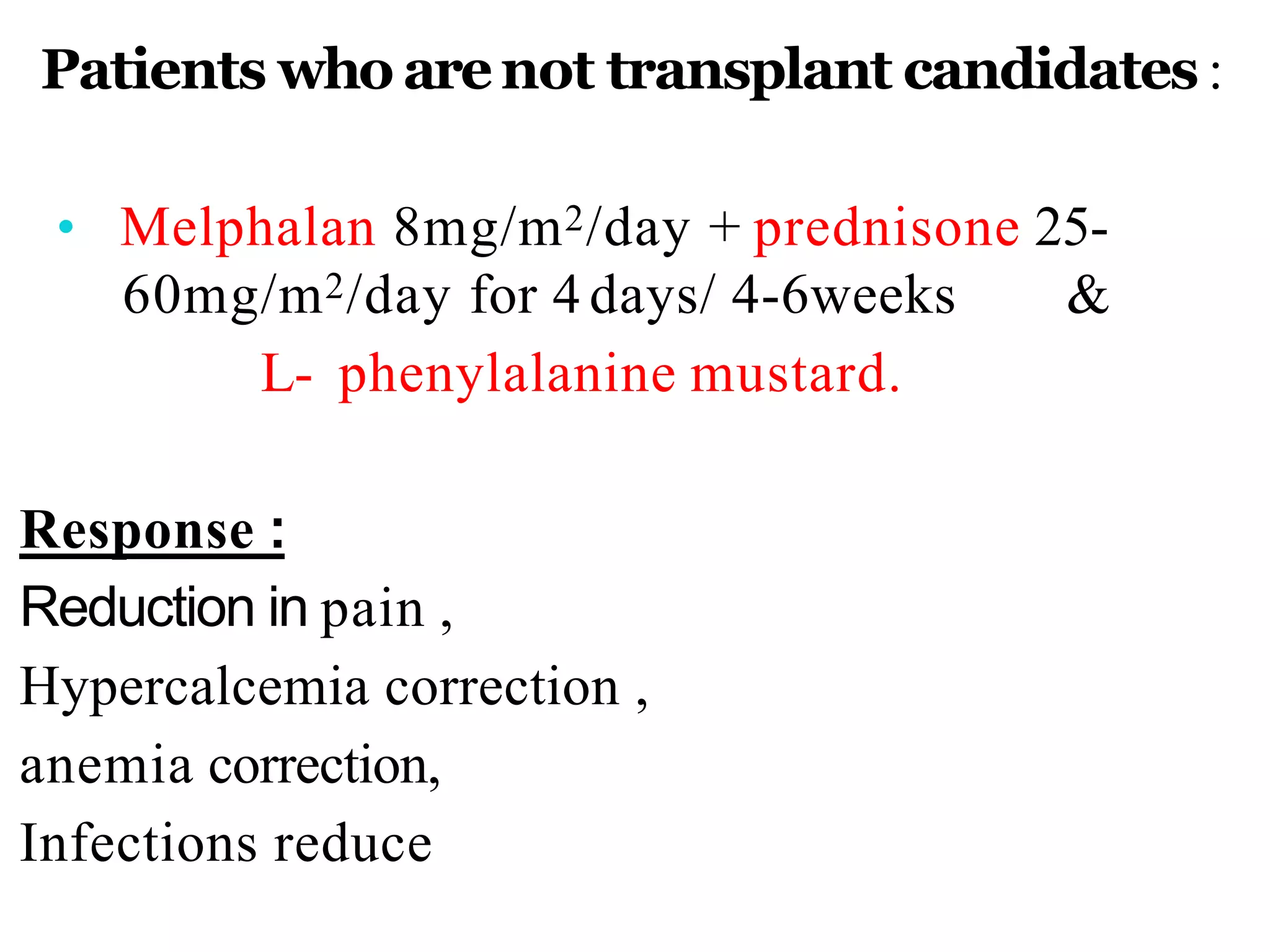
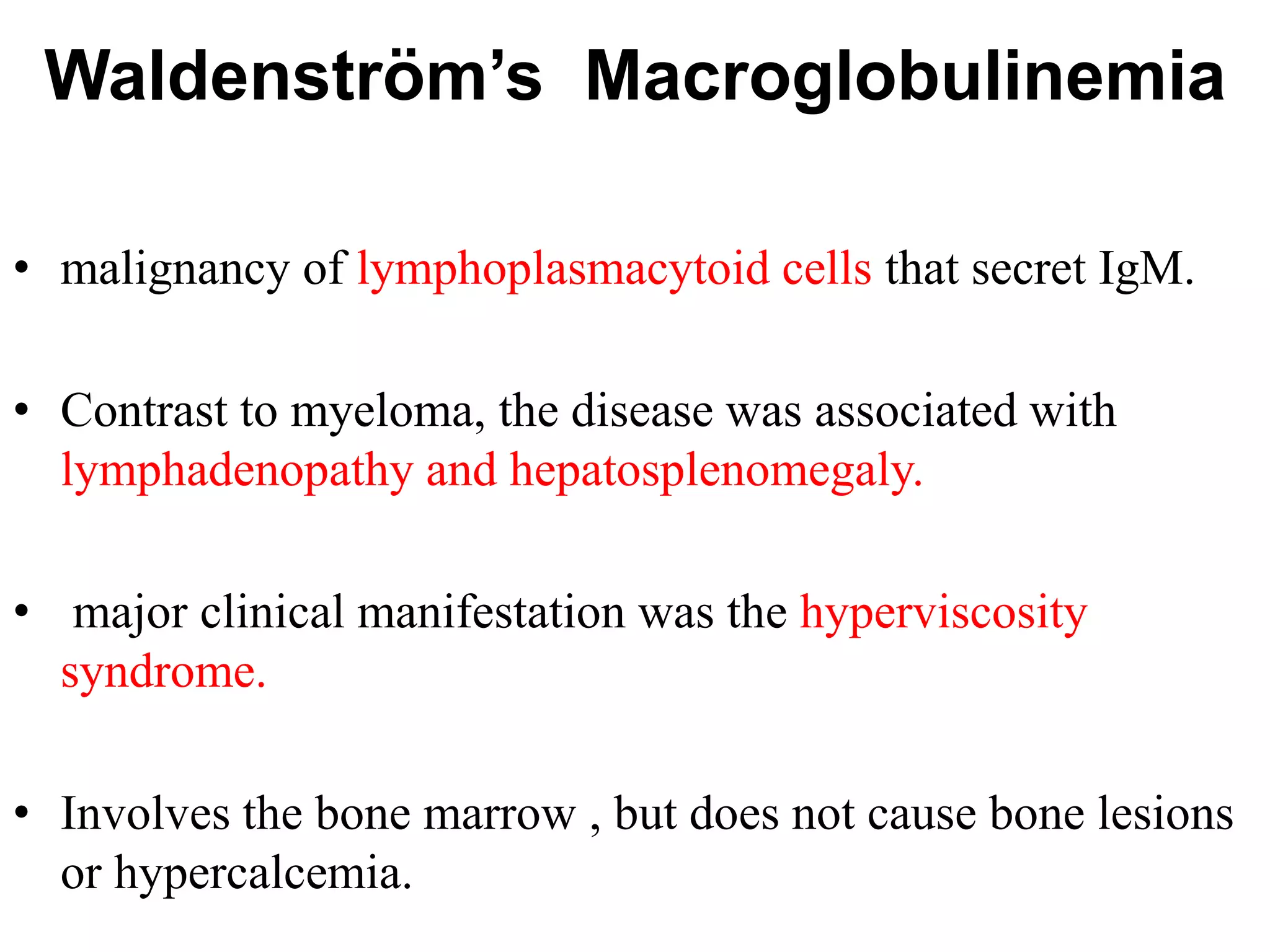
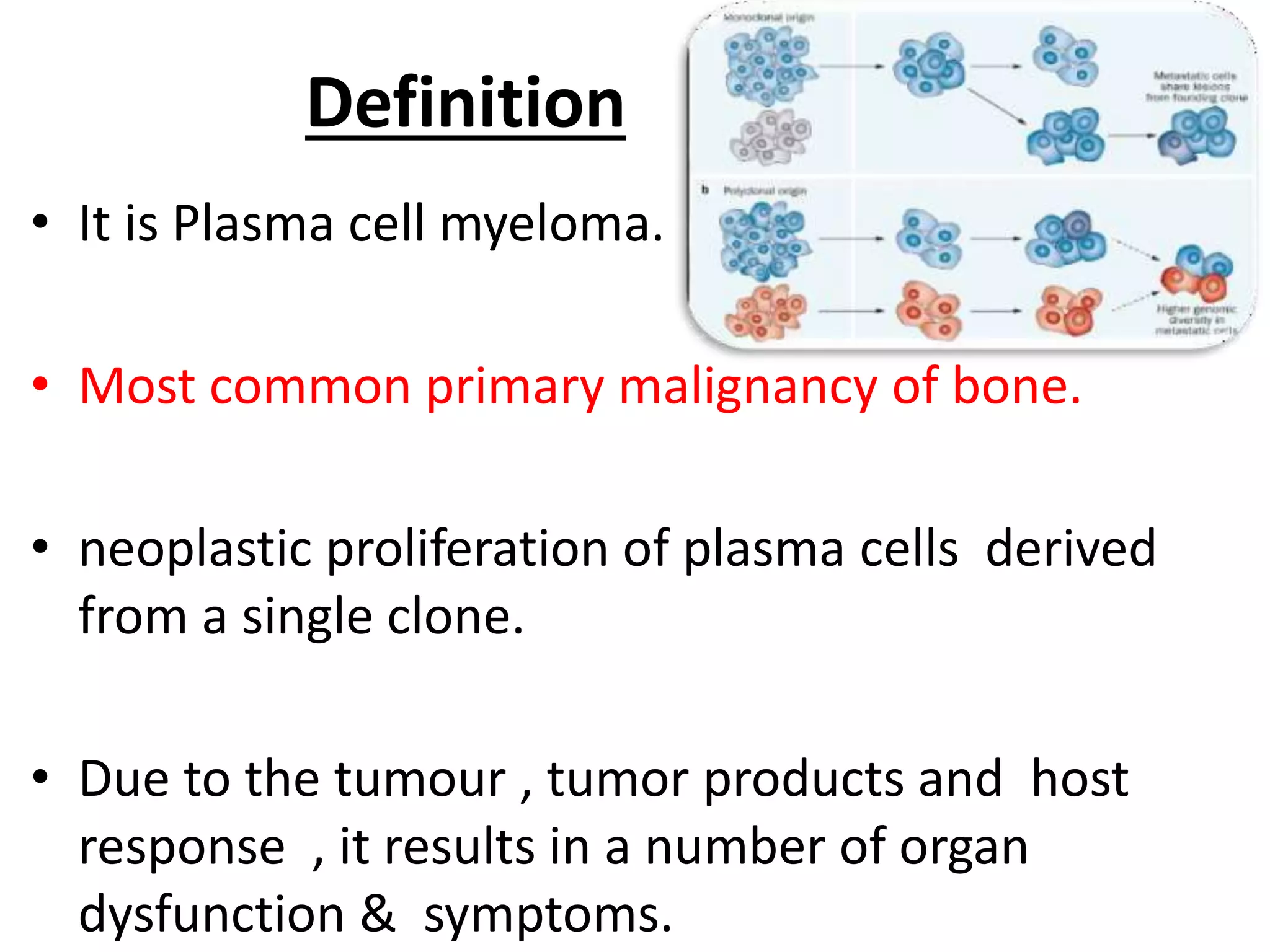
The document provides information on multiple myeloma, including its definition, history, etiology, pathogenesis, clinical presentation, investigations and staging. Some key points:
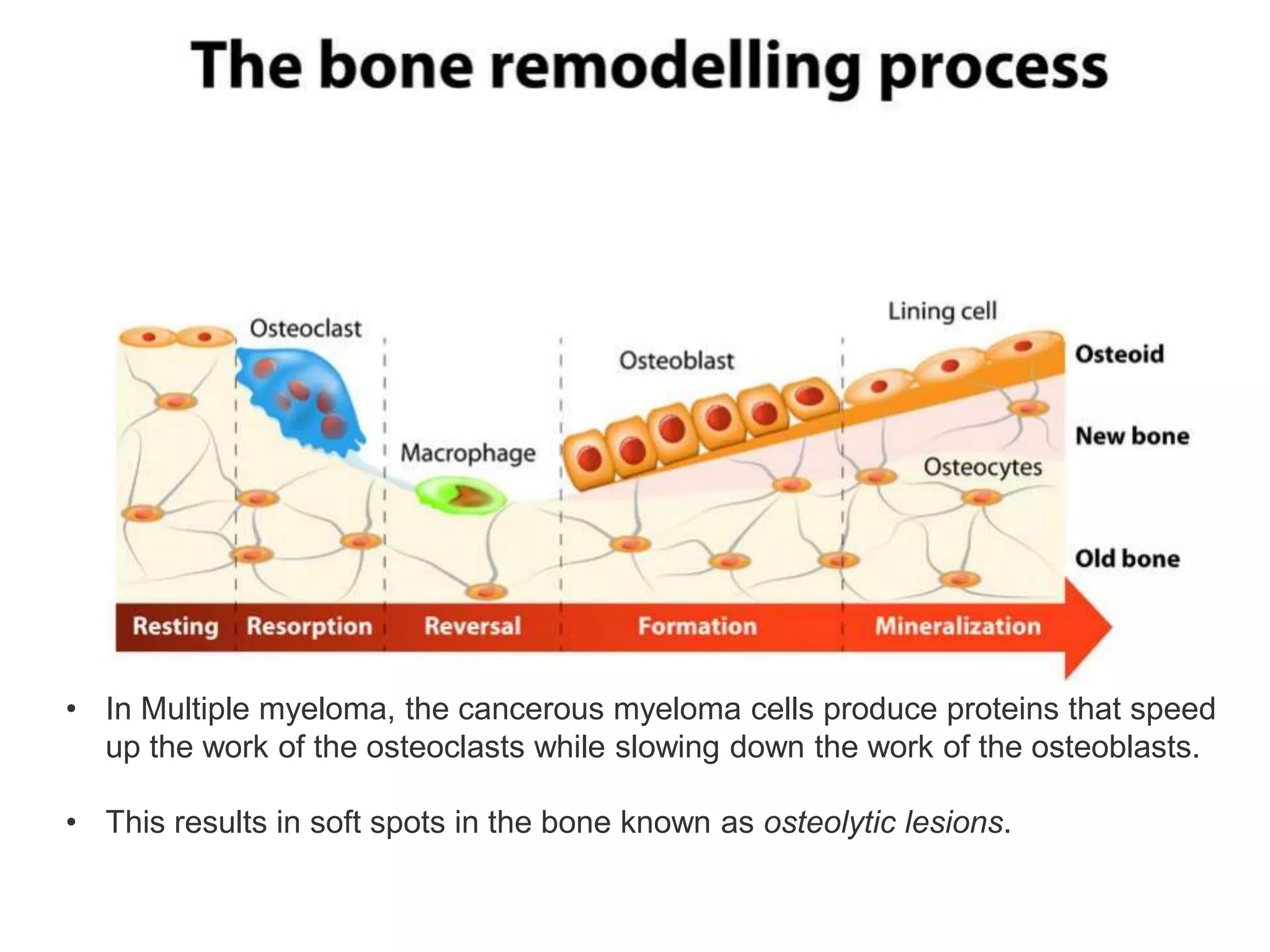
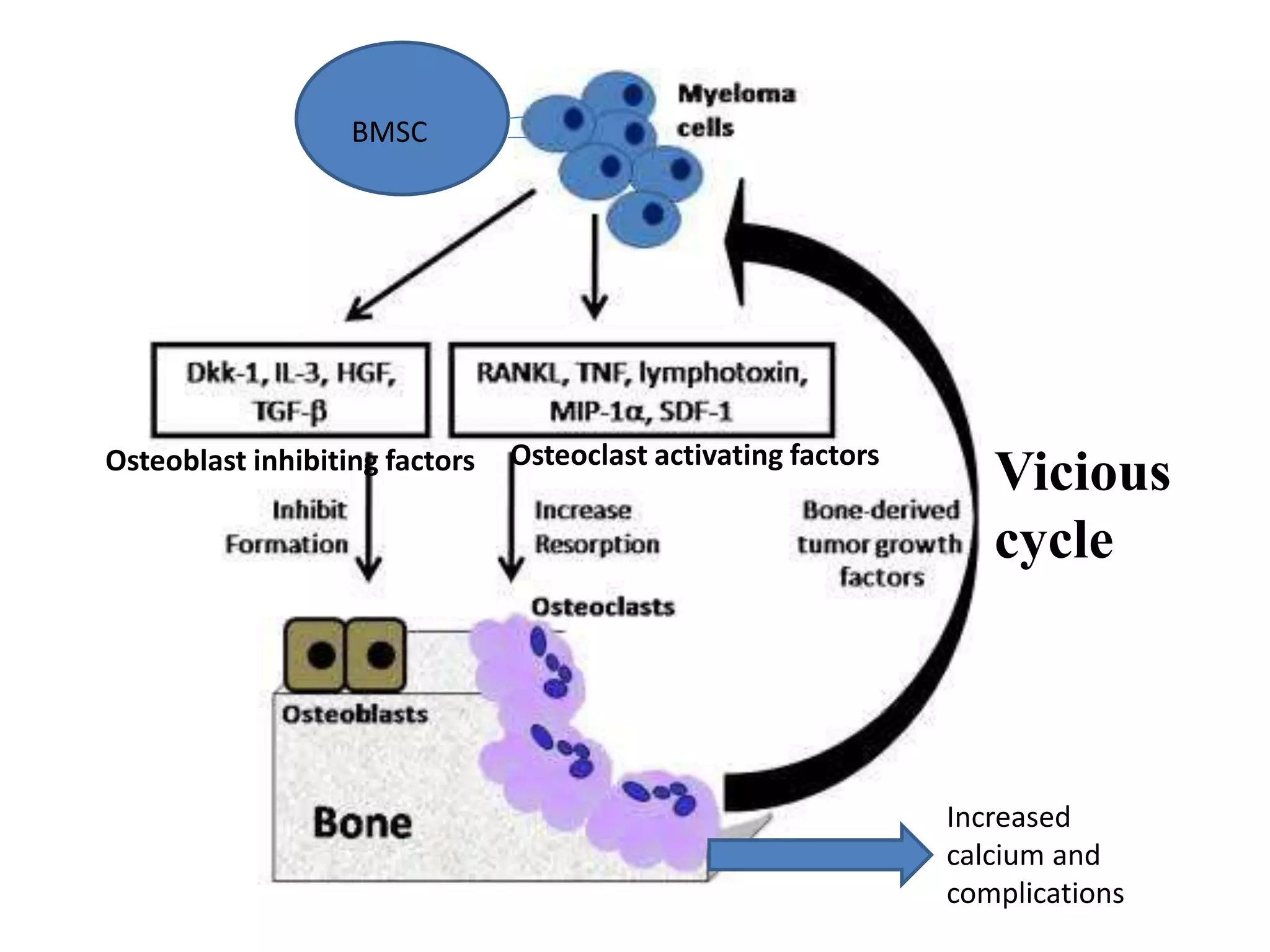
- Multiple myeloma is a neoplastic proliferation of plasma cells in the bone marrow, causing osteolytic bone lesions. It is the second most common hematologic malignancy.
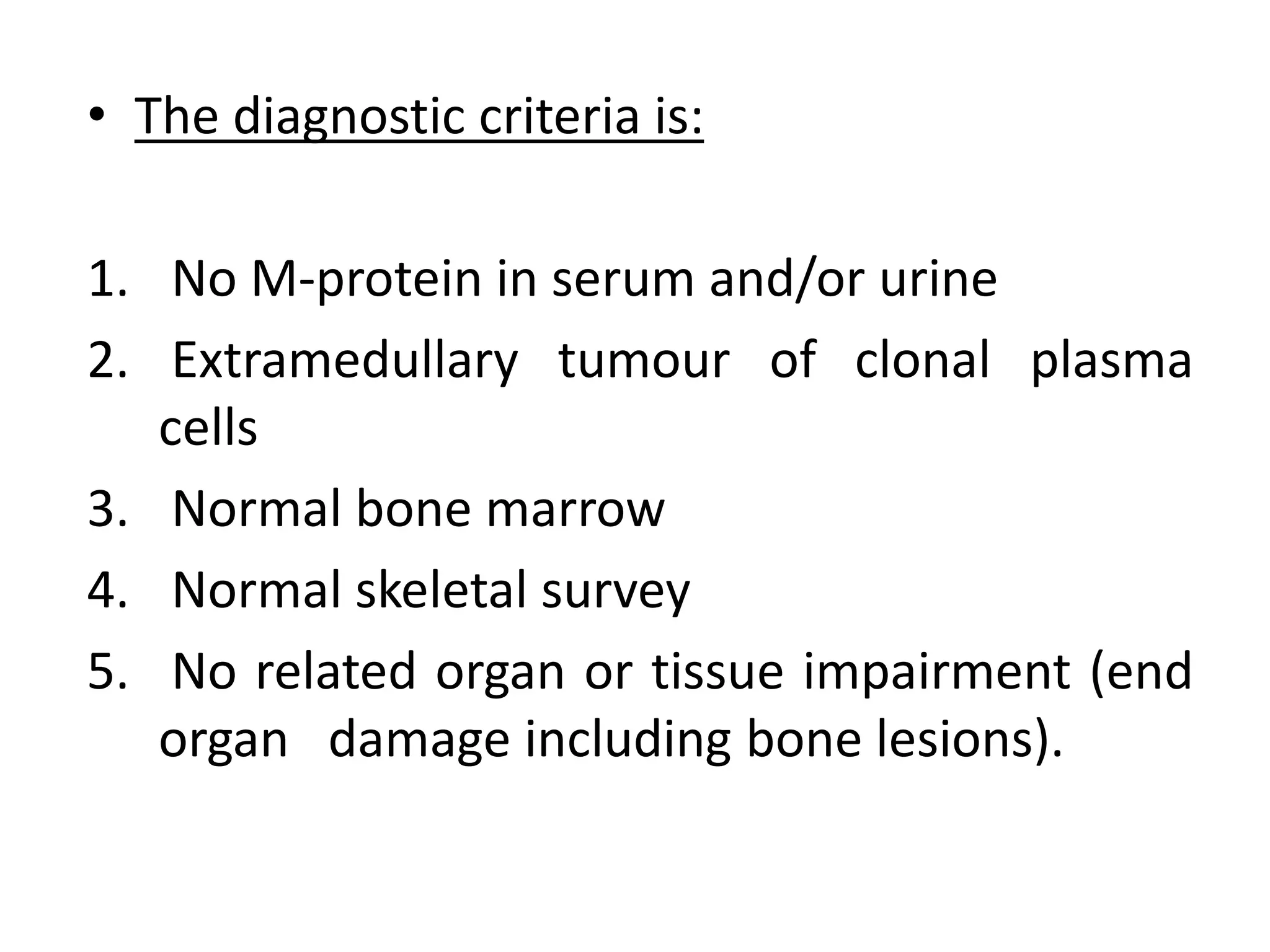
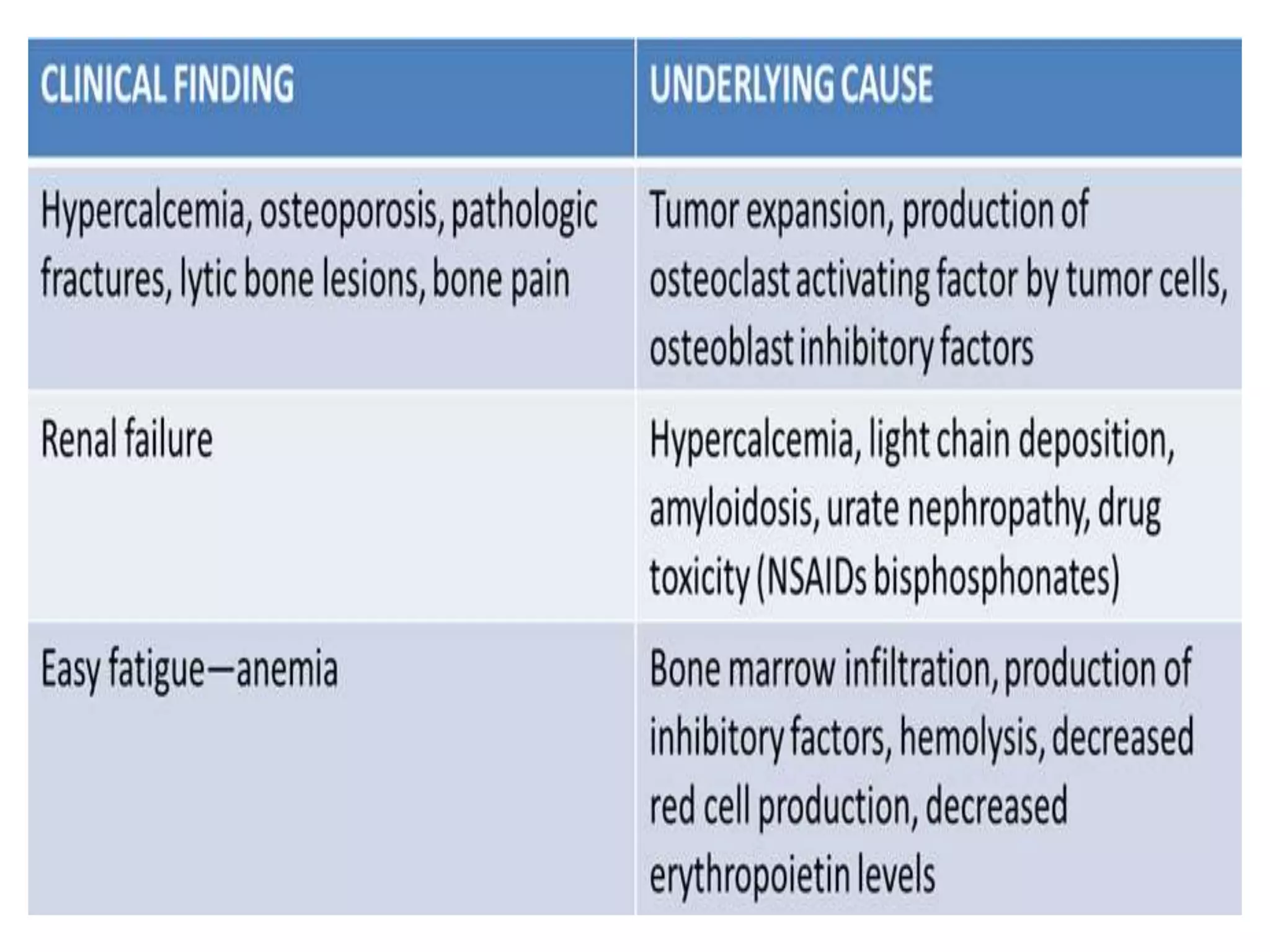
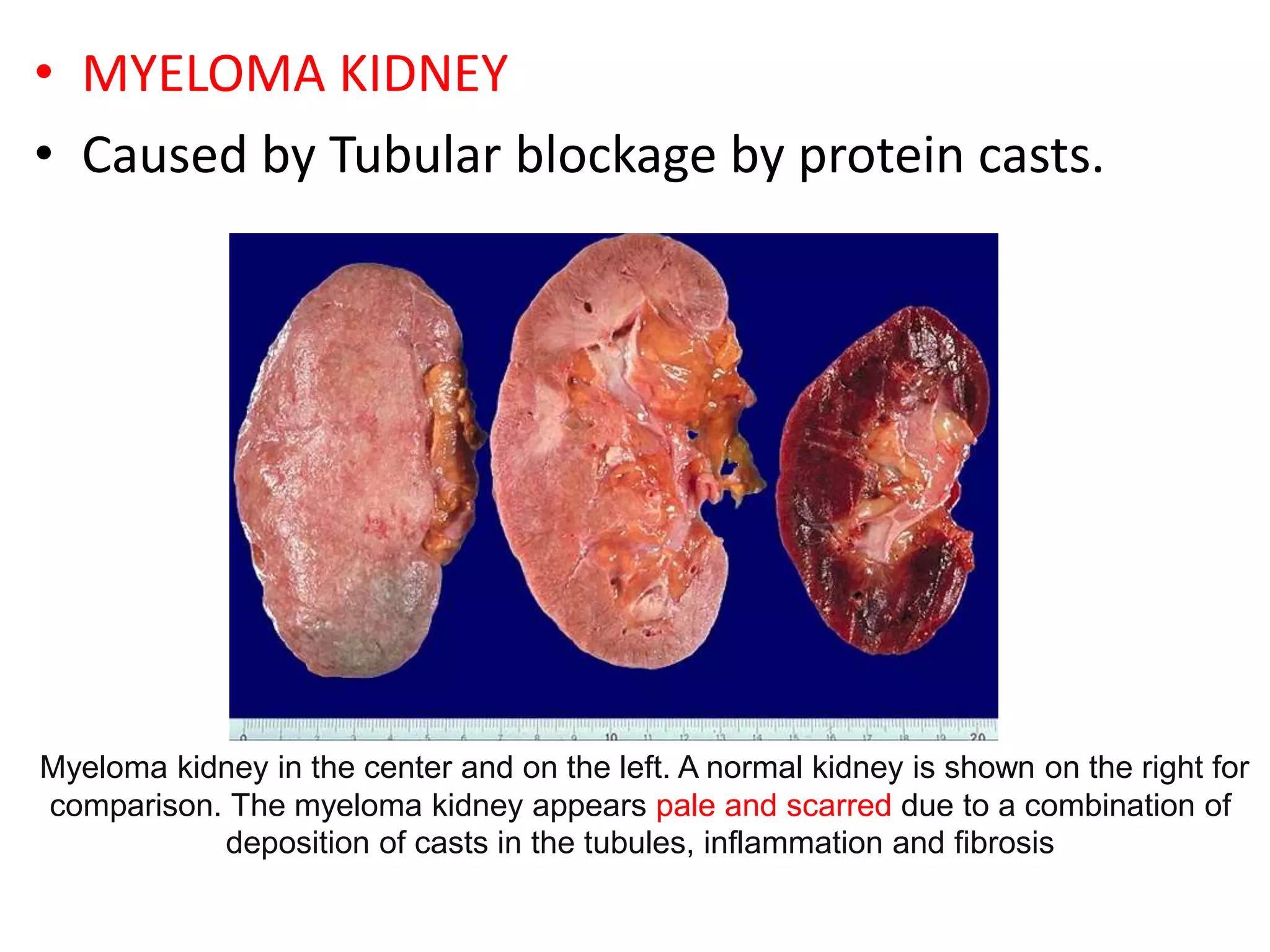
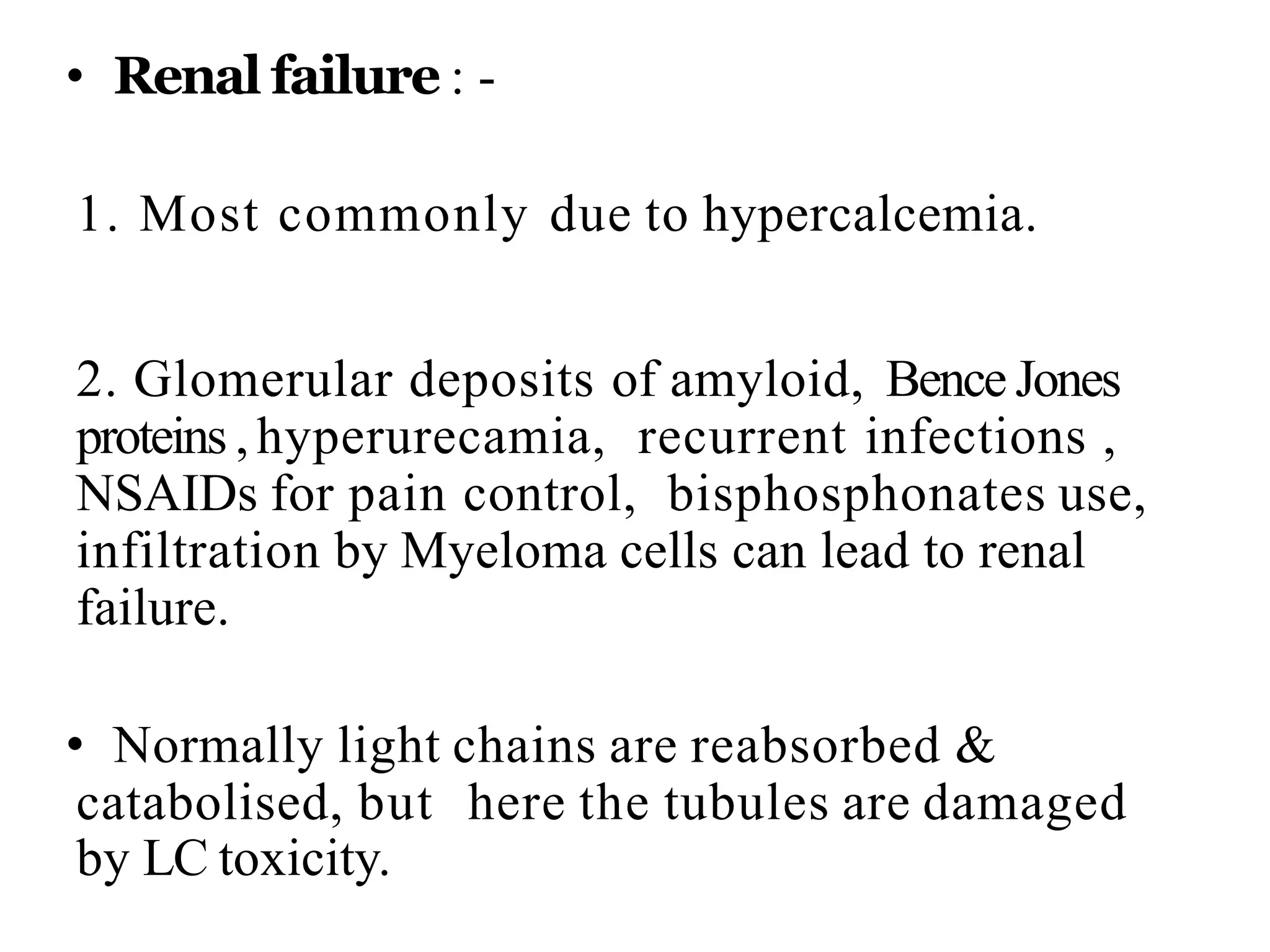
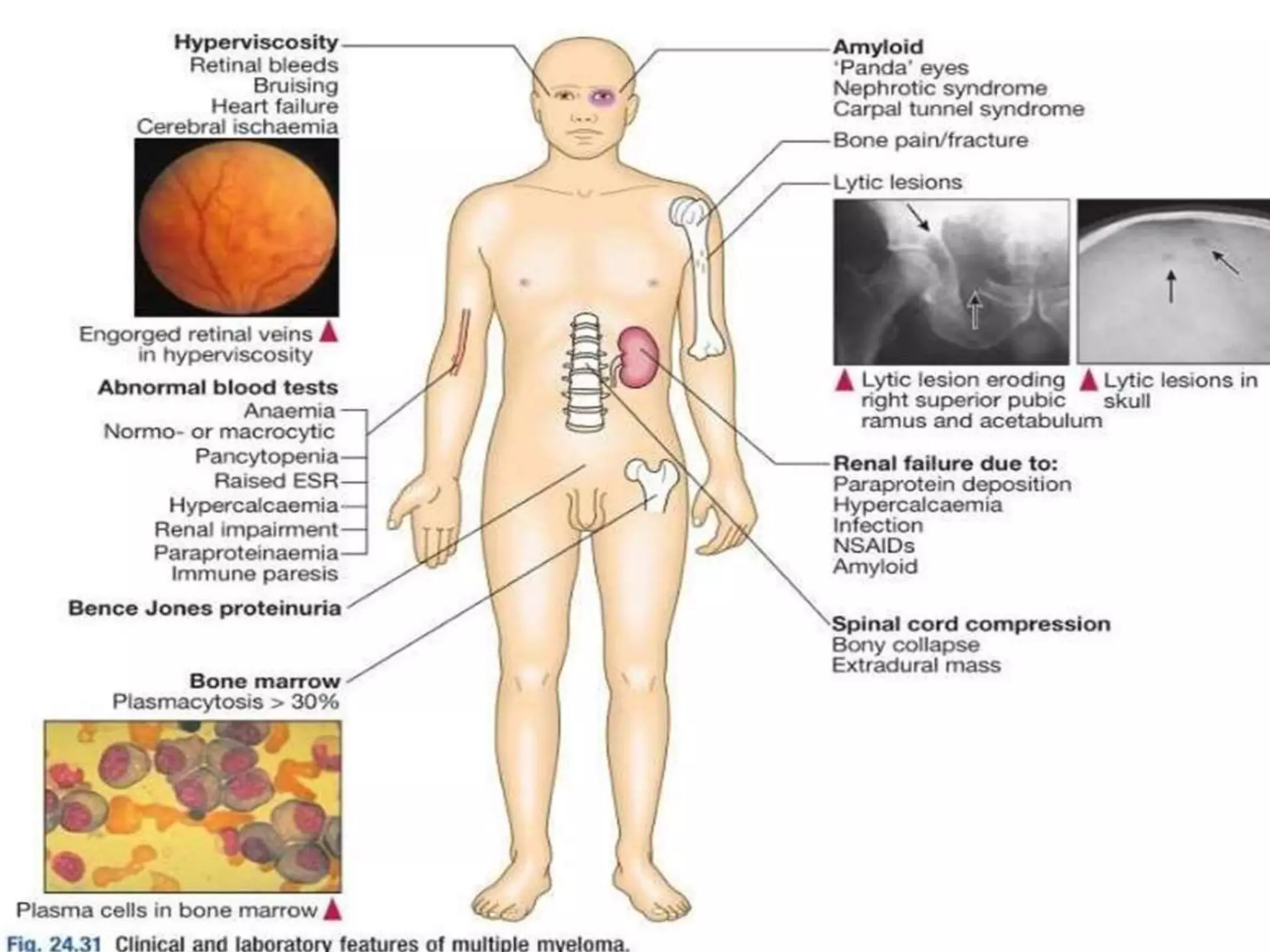
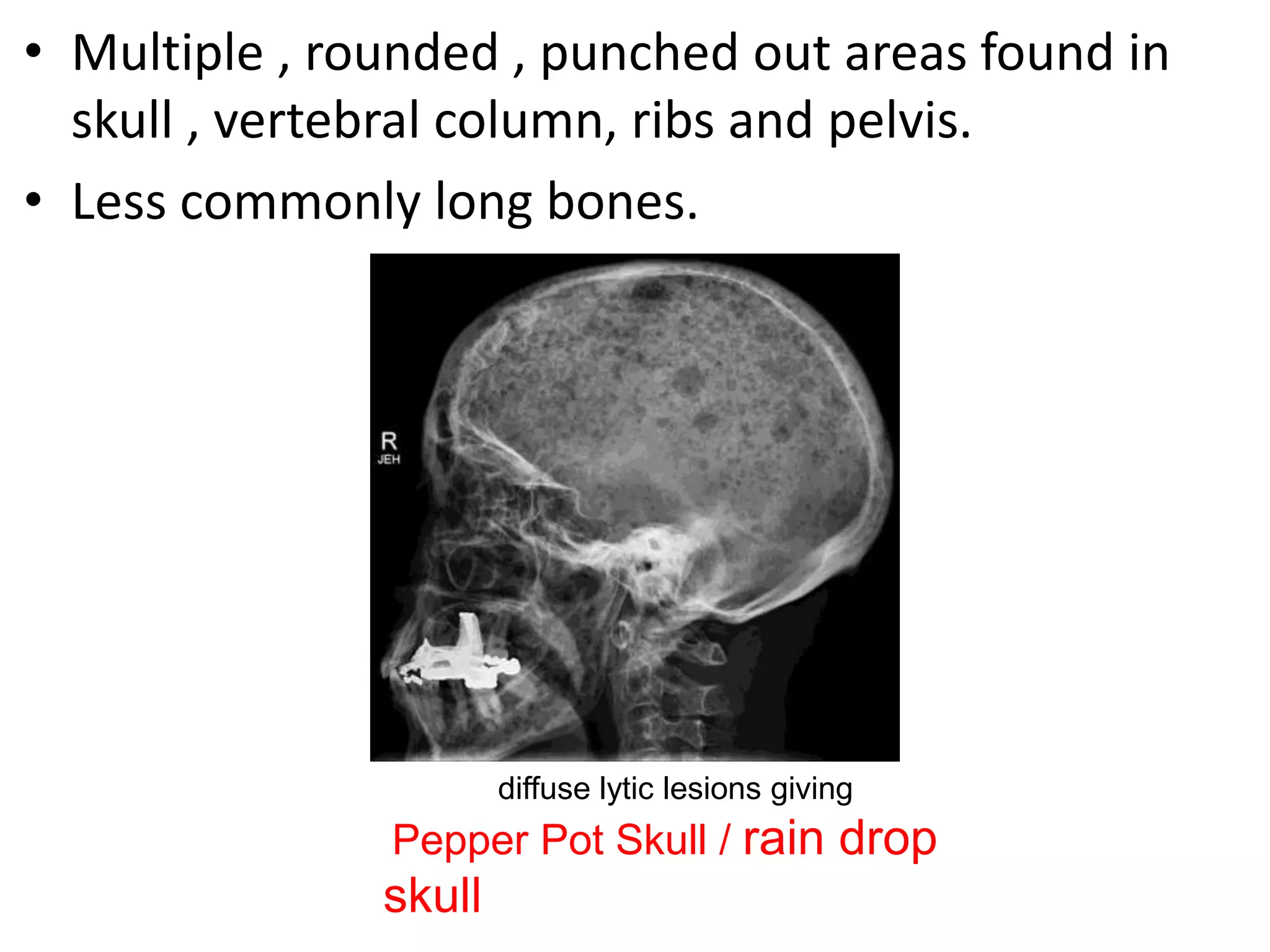
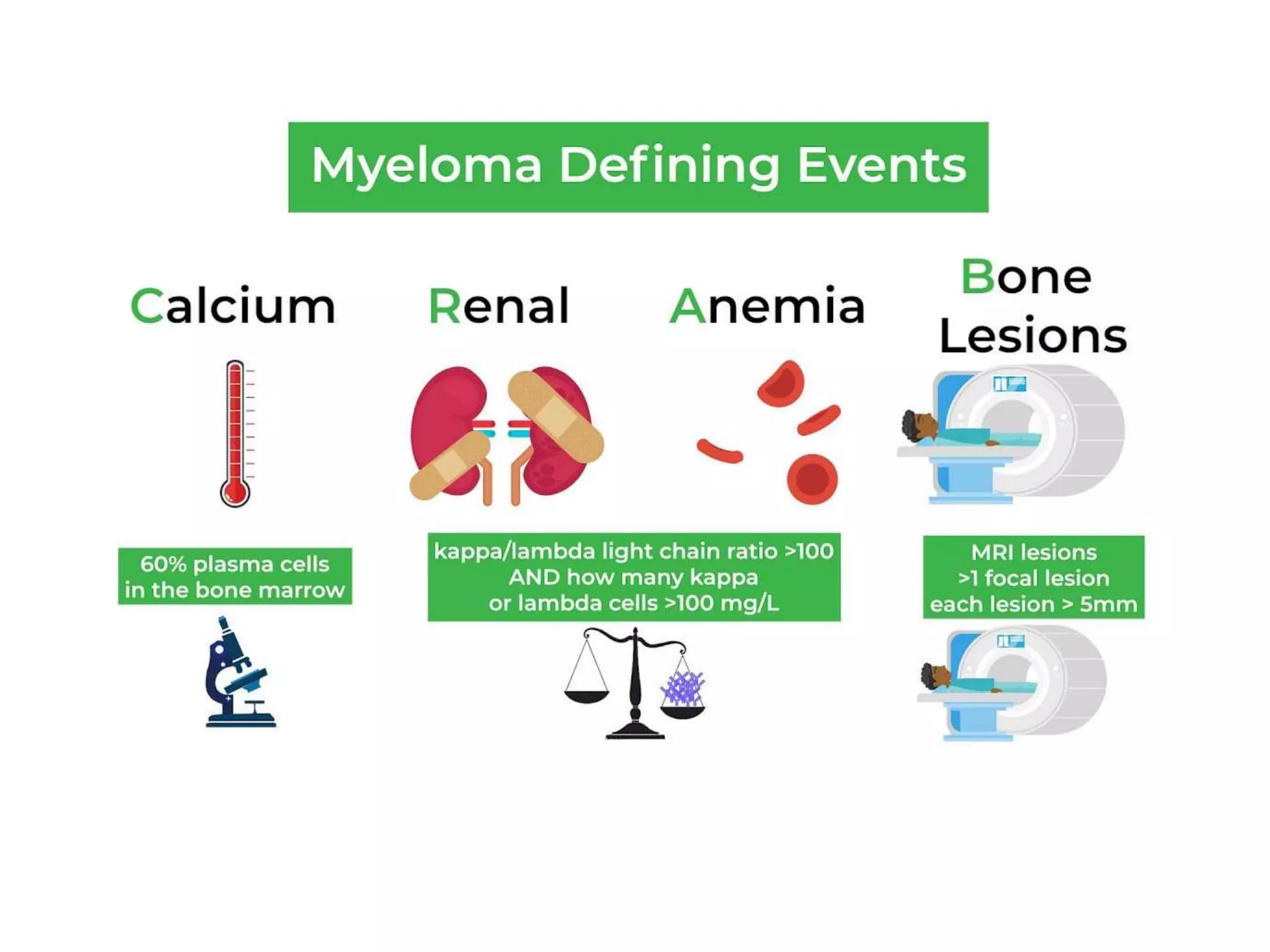
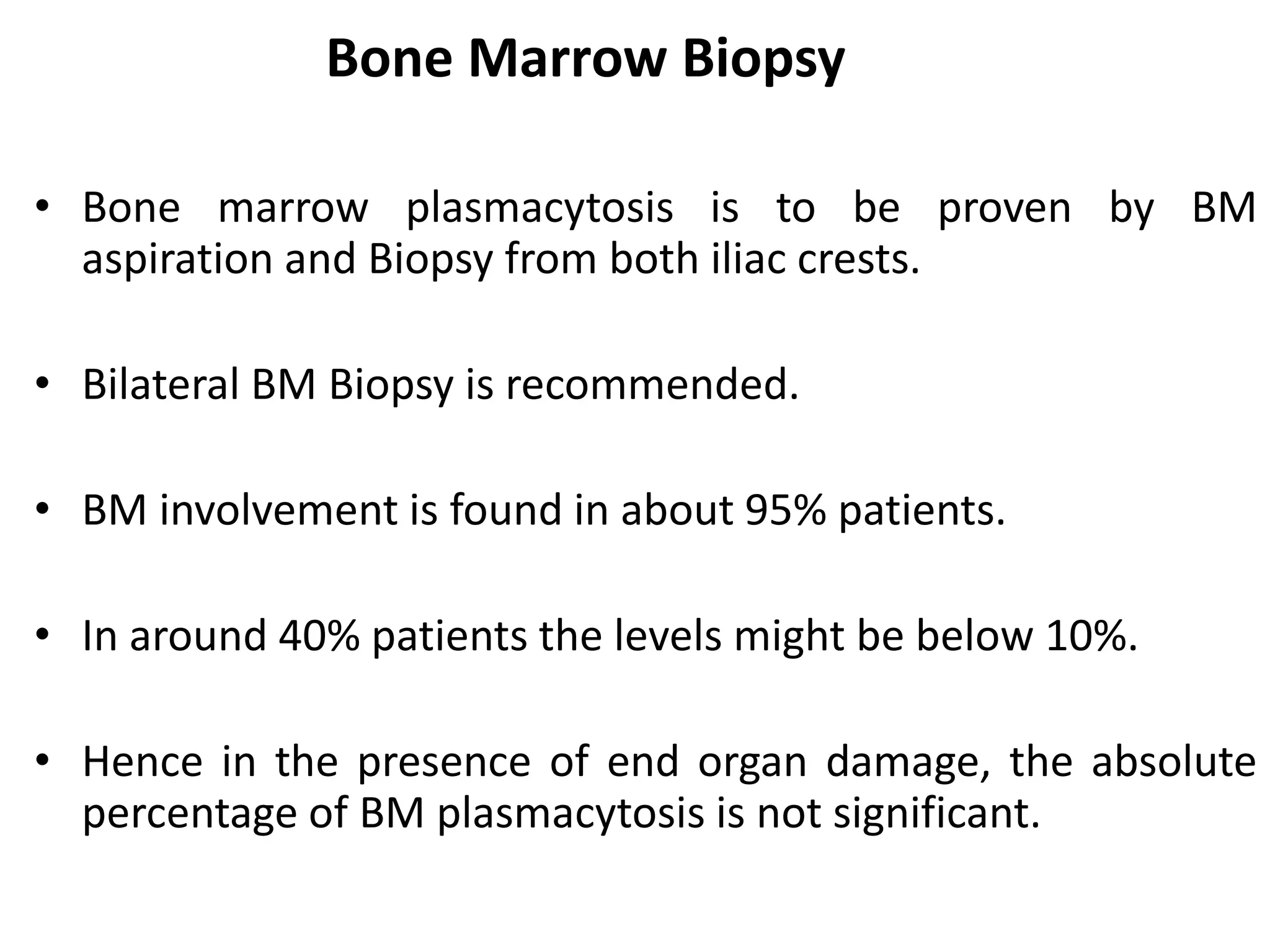
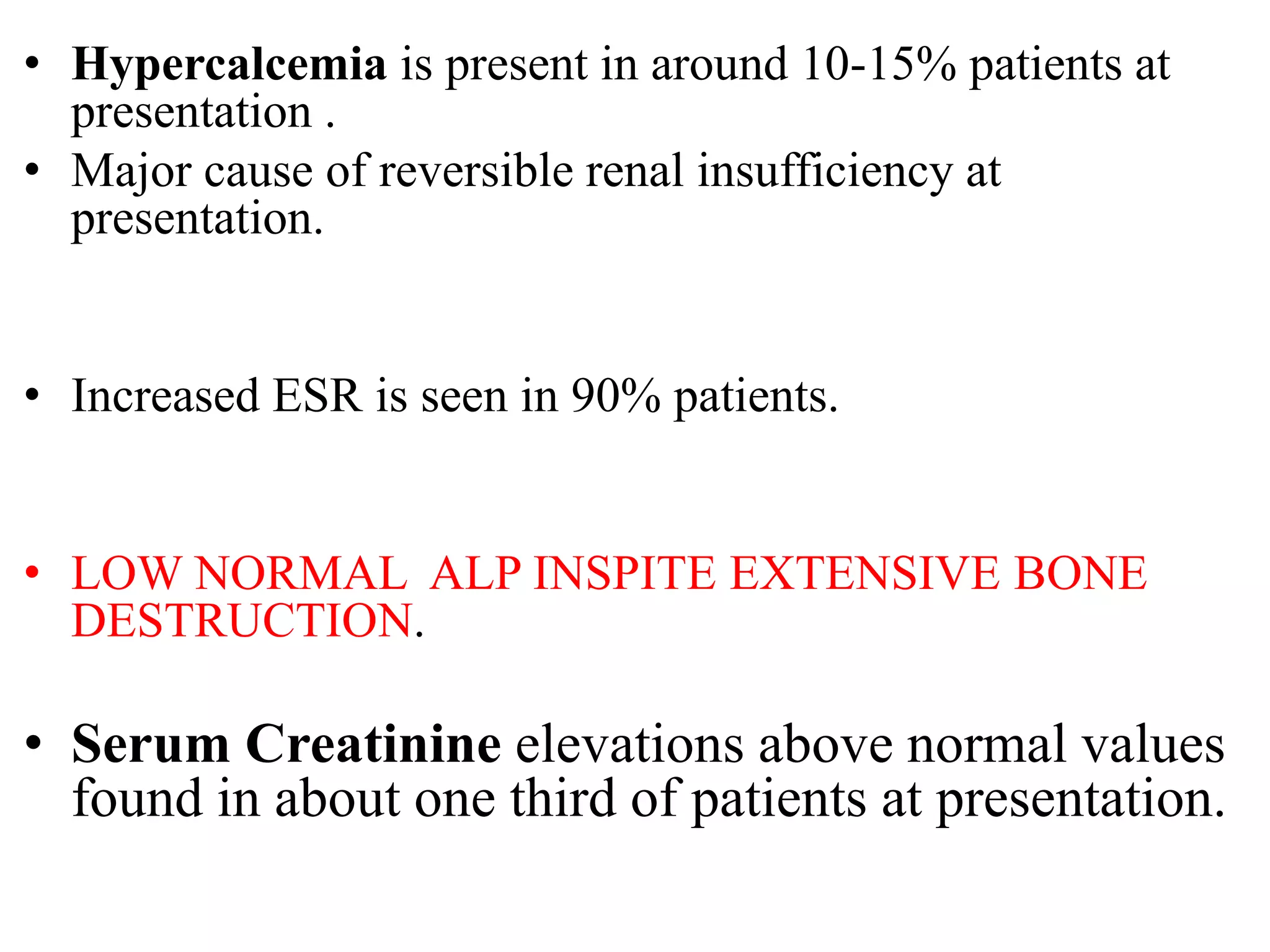
- Clinical features include bone pain, pathological fractures, anemia, hypercalcemia and renal impairment. Diagnosis requires ≥10% plasma cells on bone marrow biopsy and evidence of end organ damage.
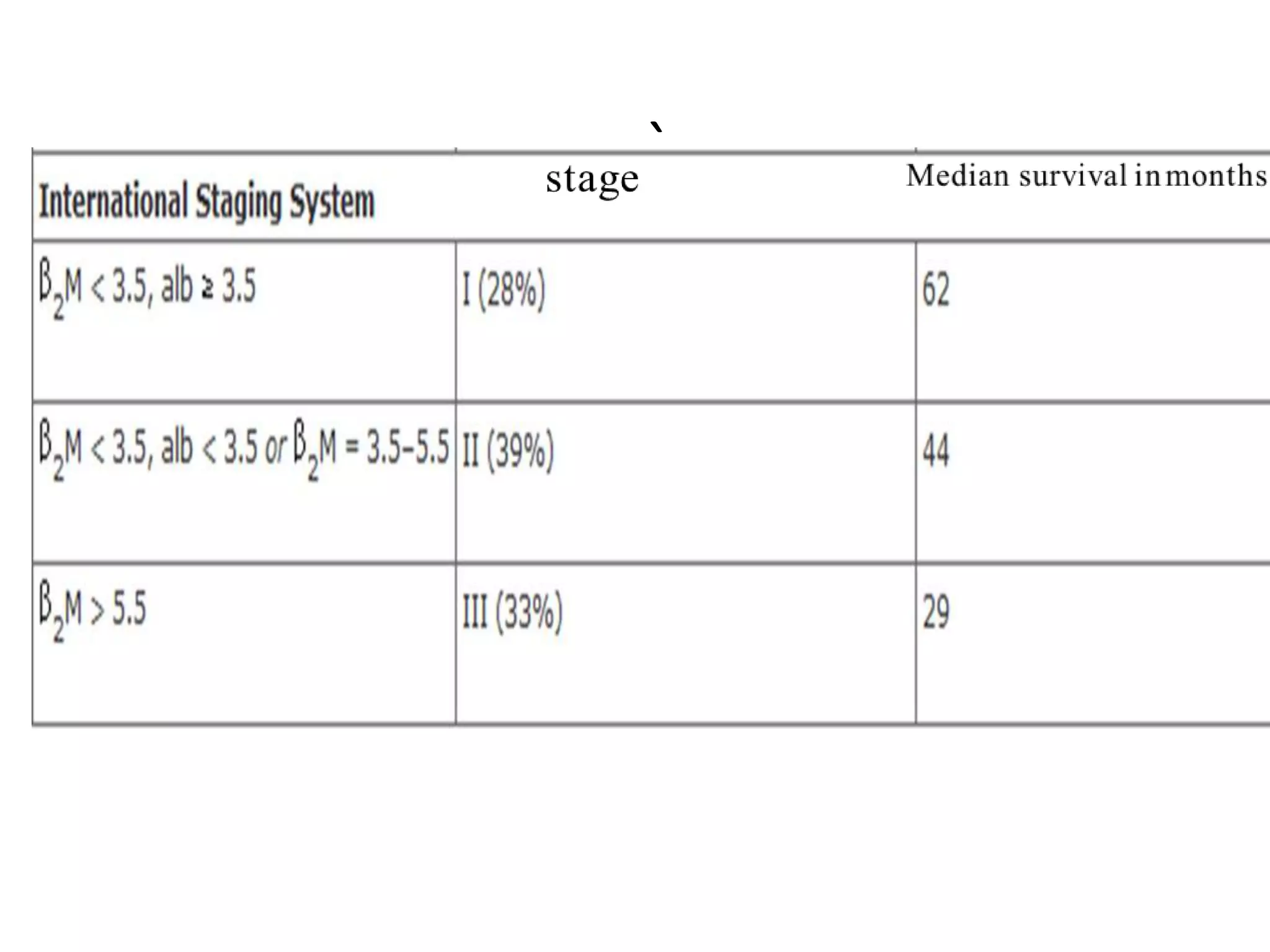
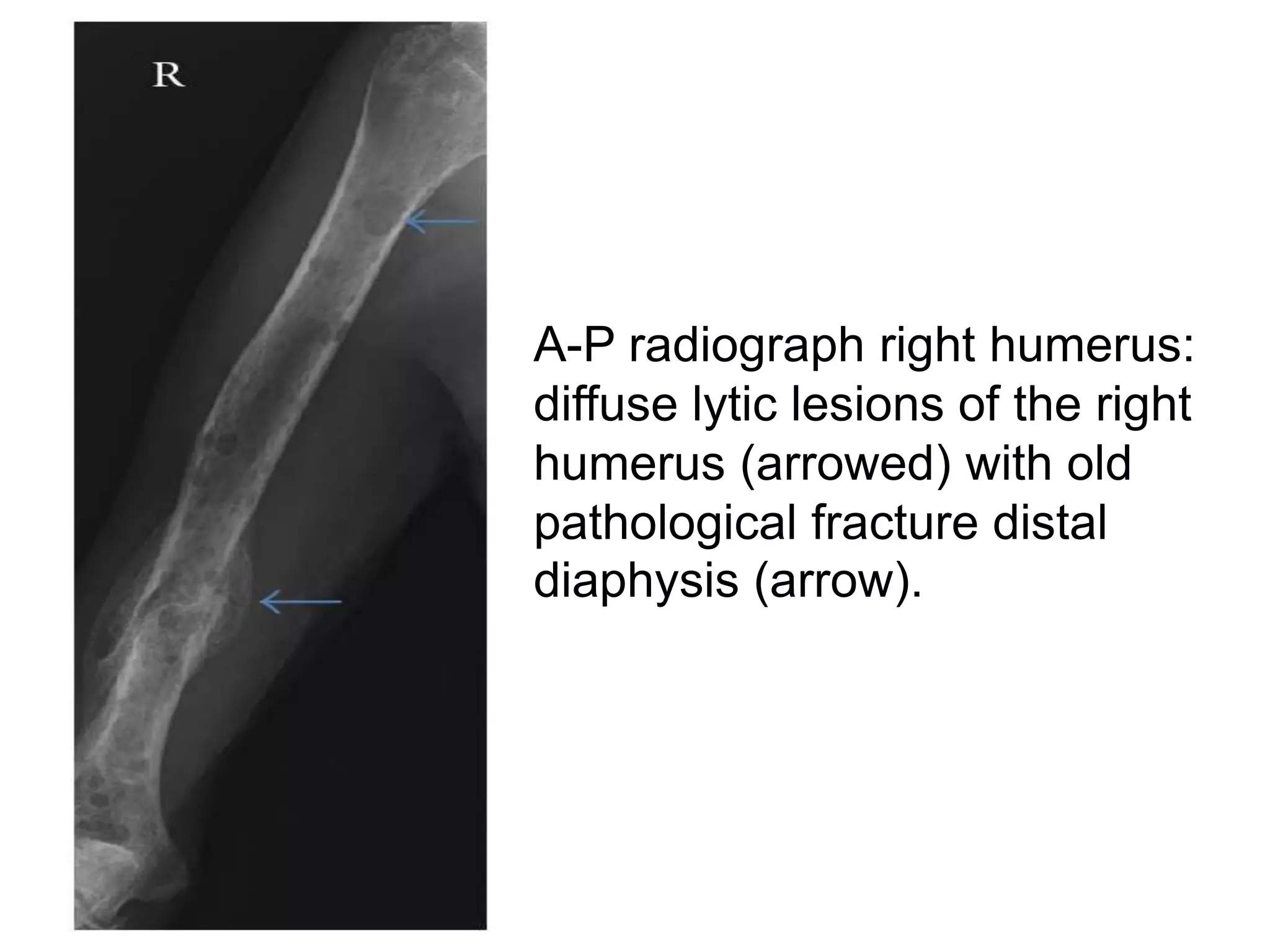
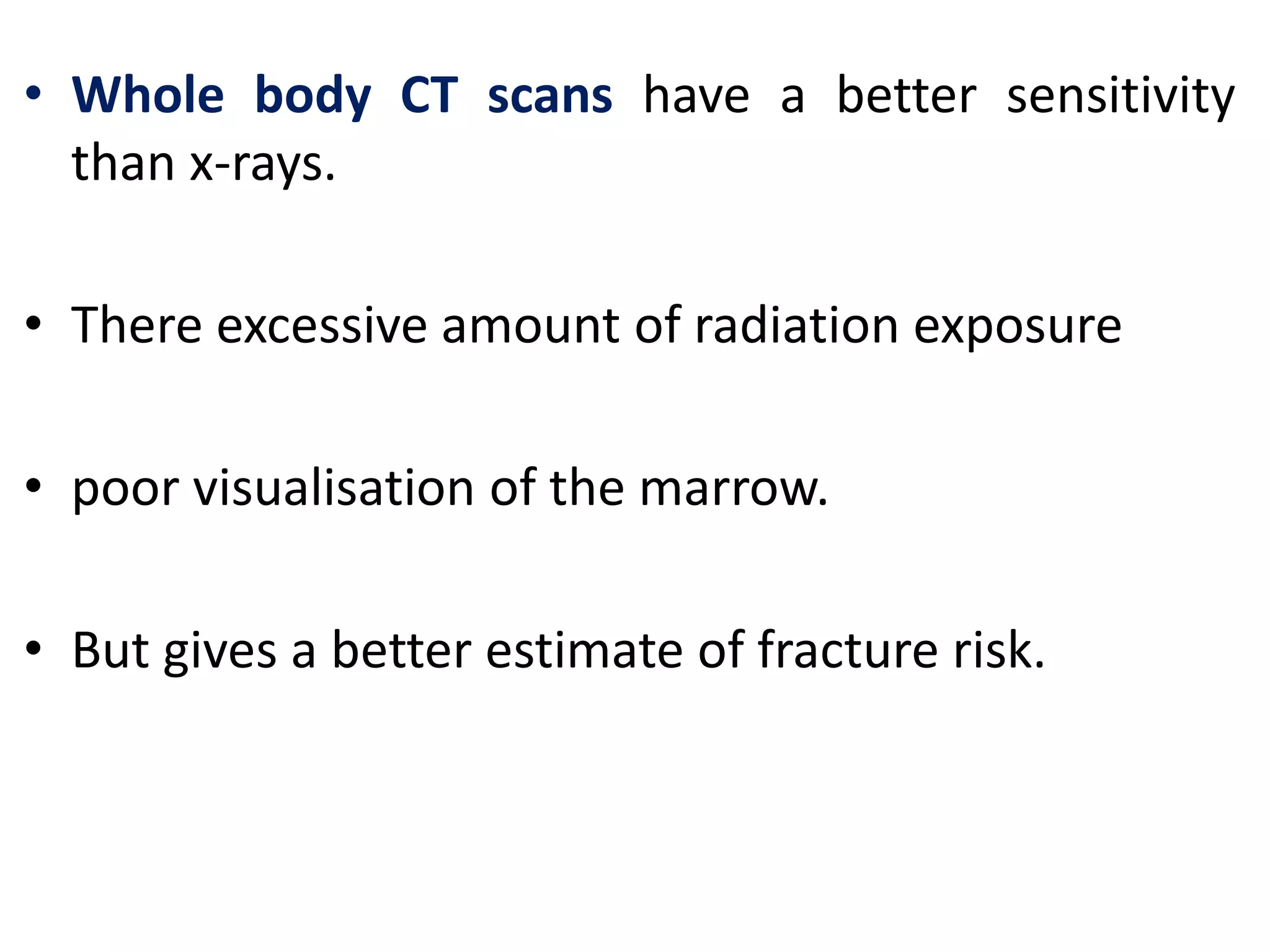
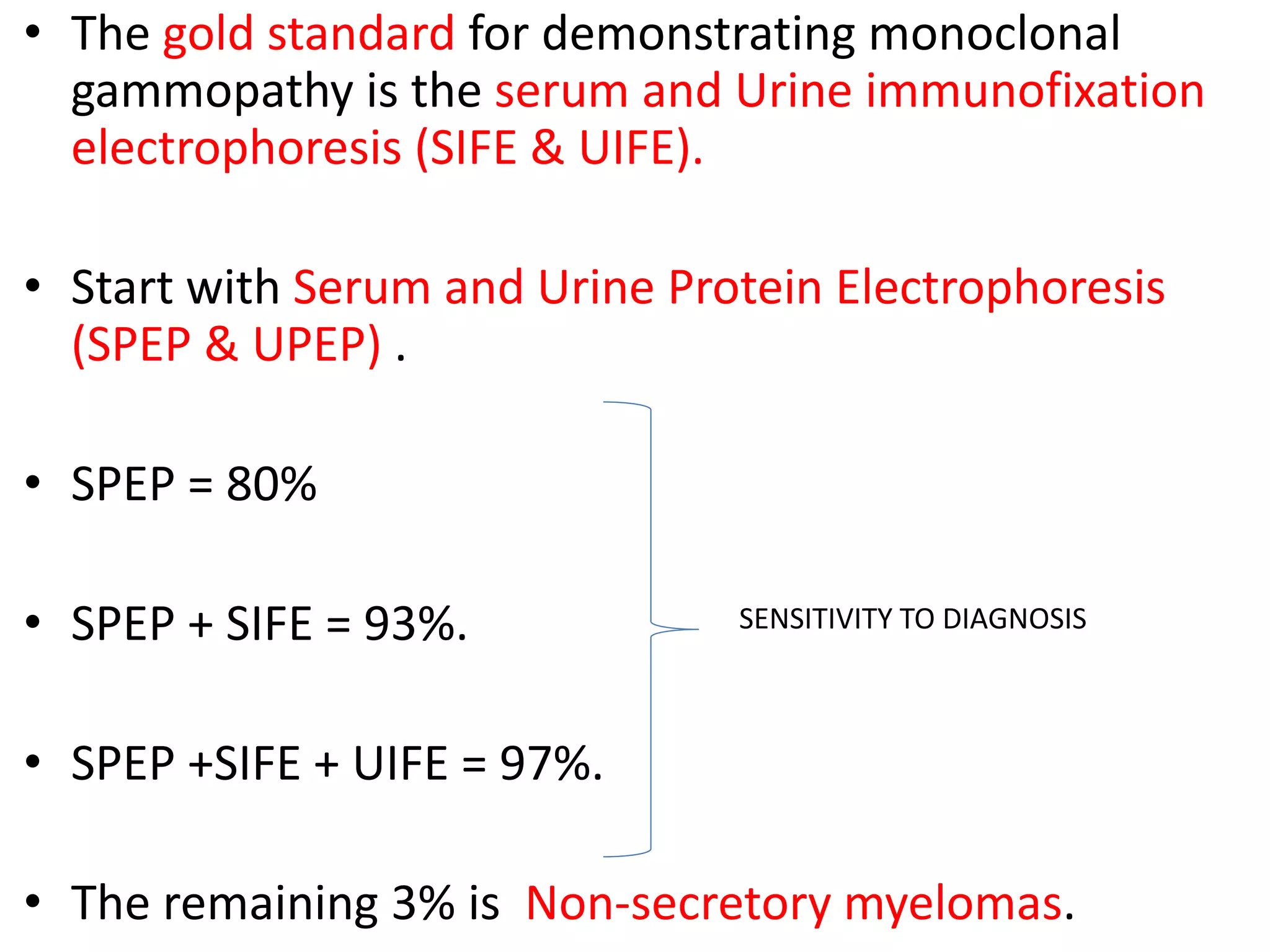
- Investigations include serum and urine protein electrophoresis, immunofixation, skeletal survey, MRI and bone marrow biopsy to assess disease burden and abnormalities. Staging involves blood










![ANEMIA:
• Normocytic , normochromic anemia seen in
~80 % pts
• Because normal marrow is replaced by
Myeloma cells.
• Reduced Hematopoiesis
Hyperviscosity syndromelike Raynauds may
develop if Myeloma component forms
cryoglobulin.[most commonly IgM,IgG3 &
IgAparaproteins]](https://image.slidesharecdn.com/multiplemyeloma-210405155651/75/Multiple-myeloma-and-its-management-21-2048.jpg)




















![Histology of MM
ECCENTRICALLY PLACED NUCLEUS + CART WHEEL [coarse chromatin]
In severe disease we can see mott cells & flame cells
FLAME CELL
Russell bodies](https://image.slidesharecdn.com/multiplemyeloma-210405155651/75/Multiple-myeloma-and-its-management-51-2048.jpg)