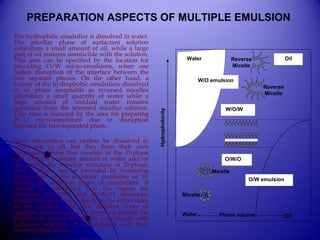
The document discusses multiple emulsions, defined as the dispersion of one immiscible liquid in another, stabilized by an emulsifying agent. It details classifications (w/o and o/w systems), types (macroemulsion, nanoemulsion, microemulsion), methods of preparation (two-step emulsification, phase inversion, membrane emulsification), and factors affecting their stability and applications in pharmaceuticals, cosmetics, and drug delivery systems. Multiple emulsions show promise for prolonged release and enhanced absorption but face limitations due to thermodynamic instability.







![Phase Inversion Technique (One step Technique)Phase Inversion Technique (One step Technique)
An increase in volume concentration of dispersed phase may cause an increase in
the phase volume ratio, which subsequently leads to the formation of multiple
emulsions.
The method typically involves the addition of an aqueous phase containing the
hydrophilic emulsifier [Tween 80/sodium dodecyl sulphate (SDS) or Cetyl
trimethyl ammonium salt (CTAB)] to an oil phase consisted of liquid paraffin and
containing lipophilic emulsifier (Span 80).
A well-defined volume of oil phase is placed in a vessel of pin mixer. An aqueous
solution of emulsifier is then introduced successively to the oil phase in the vessel
at a rate of 5 ml/min, while the pin mixer rotates steadily at 88 rpm at room
temperature. When volume fraction of the aqueous solution of hydrophilic
emulsifier exceeds 0.7, the continuous oil phase is substituted by the aqueous
phase containing a number of the vesicular globules among the simple oil
droplets, leading to phase inversion and formation of W/O/W multiple
emulsion
Aqueous phase
containing hydrophilic
emulsifier
Oil + Lipophilic
surfactant
W/O/W emulsion
Mix
b
•Volume fraction of the aqueous phase
should be higher than 0.7.
•Molar ratio of the hydrophobic and
hydrophilic emulsifier should be
optimized](https://image.slidesharecdn.com/multipleemulsion-170601130124/85/Multiple-emulsion-10-320.jpg)




![ Percent drug entrapmentPercent drug entrapment
nn10 = Initial concentration of drug in inner aqueous phase10 = Initial concentration of drug in inner aqueous phase
nn1 = Concentration of drug in dialysate1 = Concentration of drug in dialysate
VV1,1, VV2 and2 and VV0 represent the volume of inner and outer aqueous and middle oil phase0 represent the volume of inner and outer aqueous and middle oil phase
respectively, andrespectively, and VVs ands and VVd represent volume of dialyzing media and dialyzed emulsiond represent volume of dialyzing media and dialyzed emulsion
respectively.respectively.
In vitroIn vitro drug releasedrug release
The drug released from the aqueous inner phase of a W/O/W emulsionThe drug released from the aqueous inner phase of a W/O/W emulsion
can be estimated using the conventional dialysis technique.can be estimated using the conventional dialysis technique.
V = V2 +
Vd (V1+V2+V0)
Vs
C = 100/[1-n1V*/n10-n1)V1]
Water out
Water in
Receptor compartment
Dialysis bag
Sampling pipette
Temperature jacket](https://image.slidesharecdn.com/multipleemulsion-170601130124/85/Multiple-emulsion-15-320.jpg)


