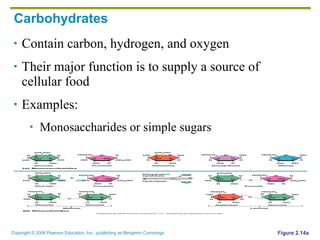
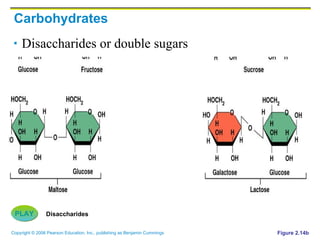
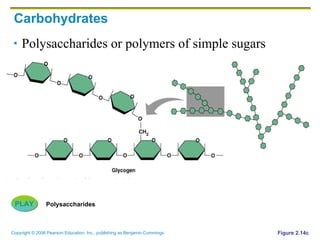
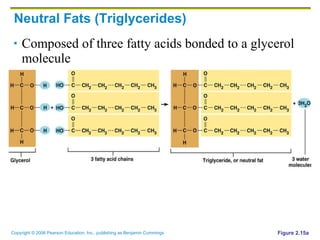
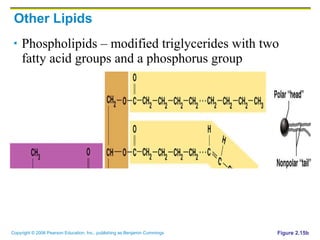
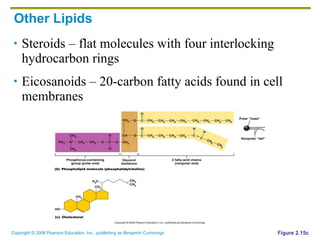
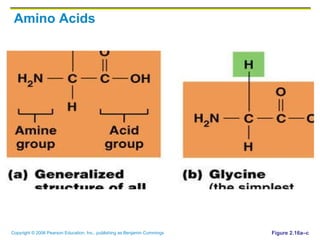
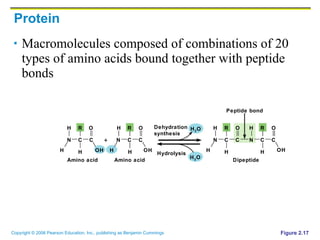
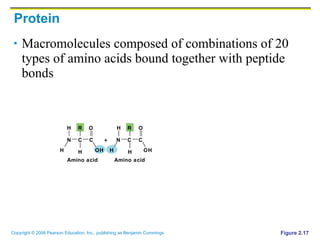
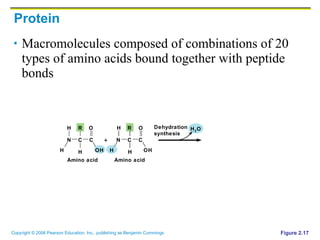
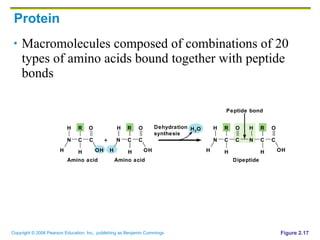
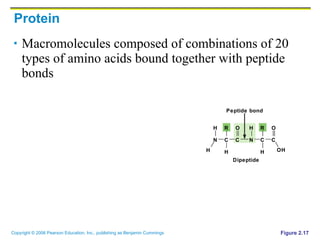
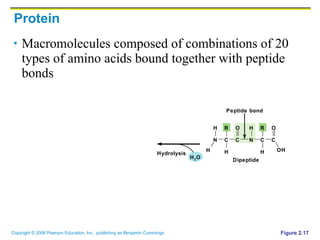
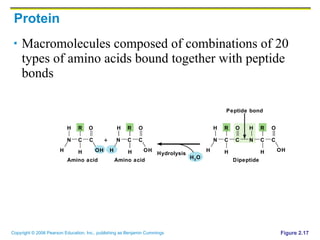
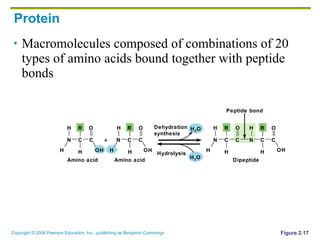
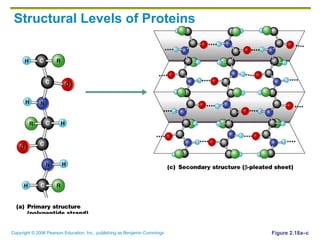
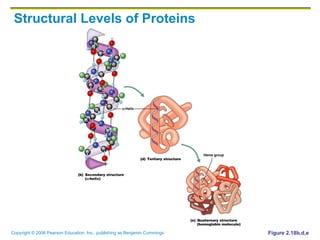
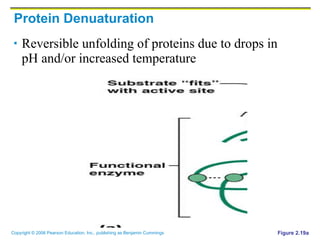
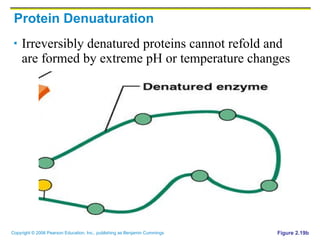
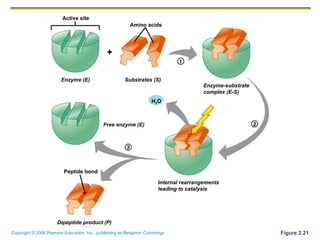
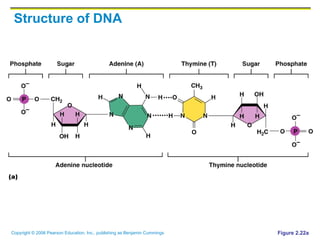
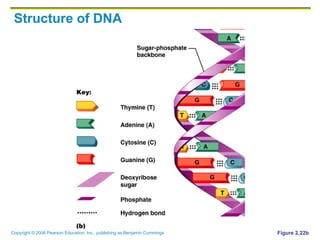
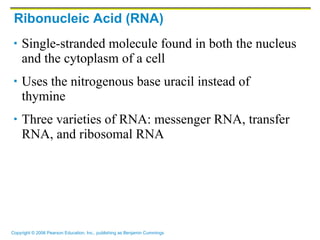
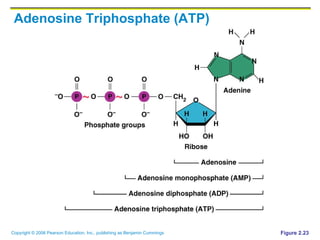
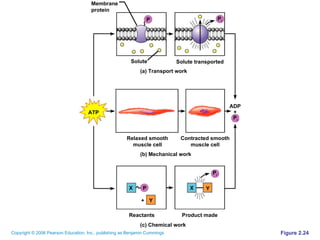
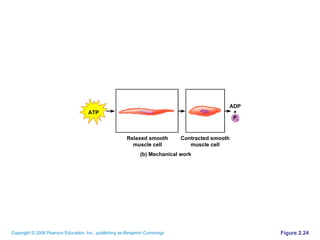
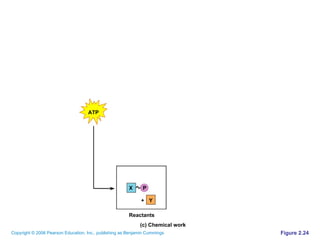
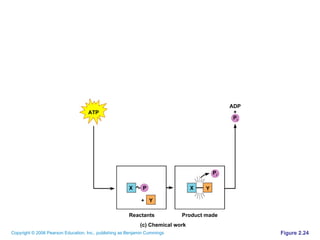
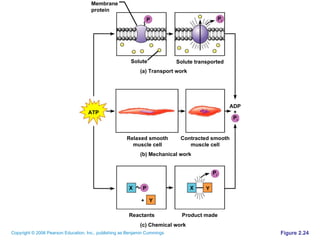
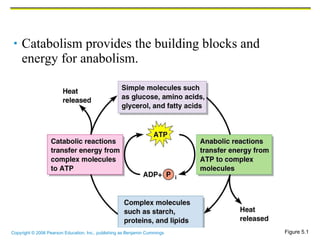
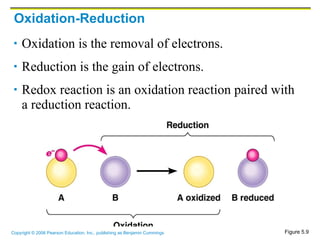
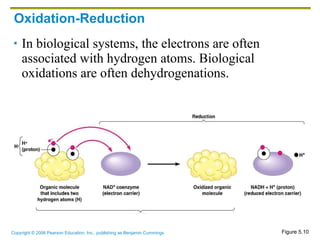
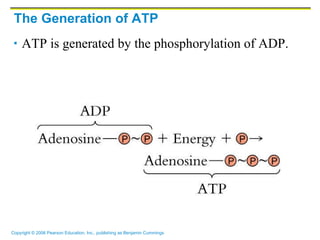
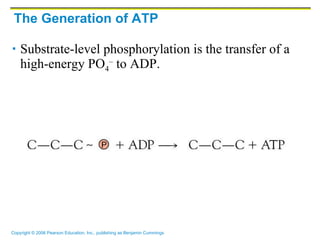
The document discusses the main types of biological macromolecules - proteins, carbohydrates, lipids, and nucleic acids. It provides details on their structures, functions and examples of each type of macromolecule. The key macromolecules discussed are proteins, which are composed of amino acids, and nucleic acids like DNA and RNA, which provide genetic instructions and are made of nucleotides containing nitrogen bases. Energy production in cells is also summarized, with ATP being generated through substrate-level phosphorylation or chemiosmosis using electron transport chains.