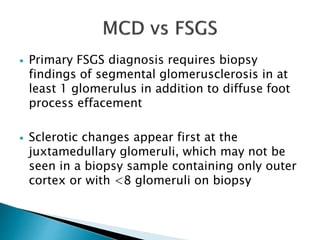
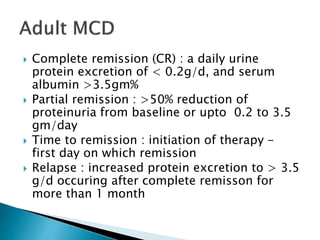
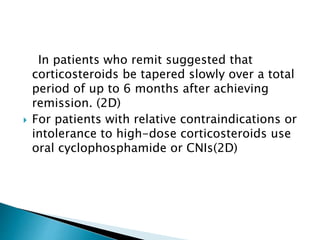
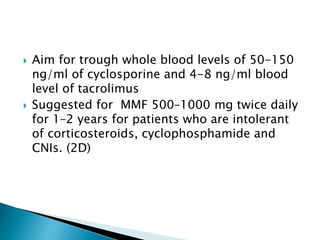
This document summarizes minimal change disease (MCD), including its pathogenesis, clinical presentation, diagnosis, treatment and management. Some key points:
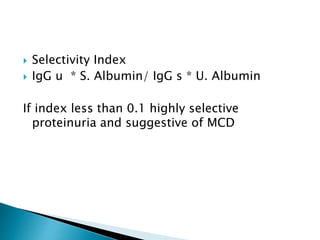
- MCD is the most common cause of nephrotic syndrome in children and adults. It involves injury to the podocytes resulting in proteinuria.
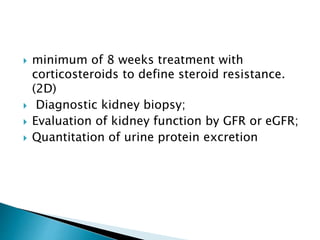
- Clinically it presents with nephrotic syndrome - heavy proteinuria, hypoalbuminemia, edema, and hyperlipidemia. Renal function is usually normal.
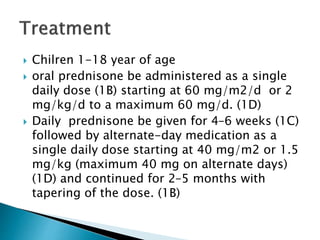
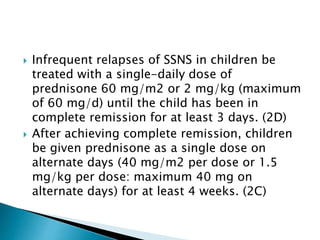
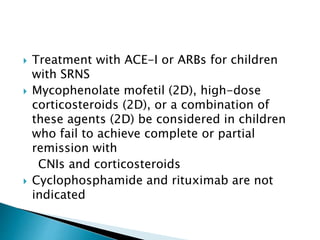
- Treatment of initial episodes involves corticosteroids, which induce remission in 80% of adults. Relapses are common and additional immunosuppressants may be needed.
- Management of relapses and frequent rel