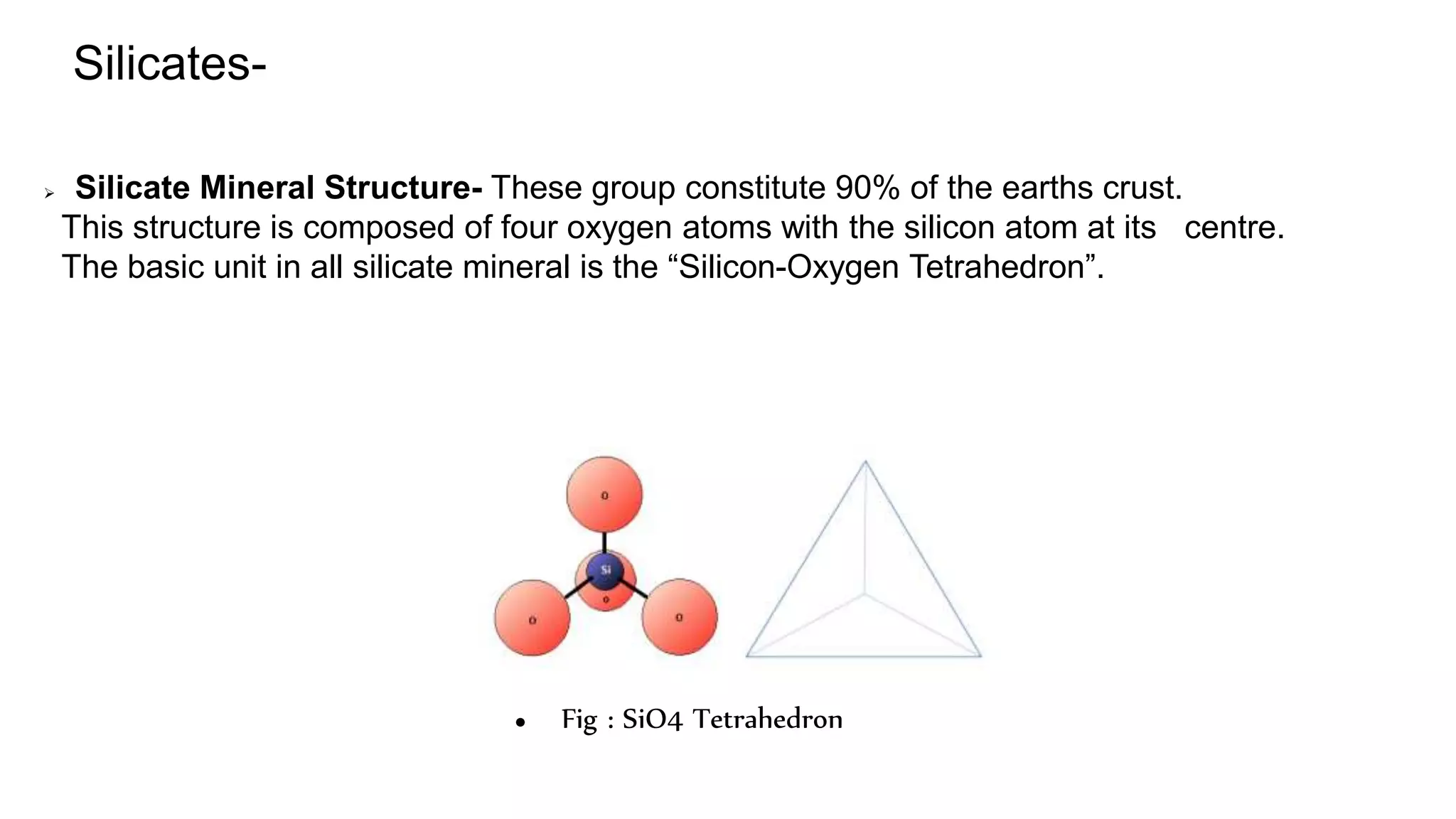
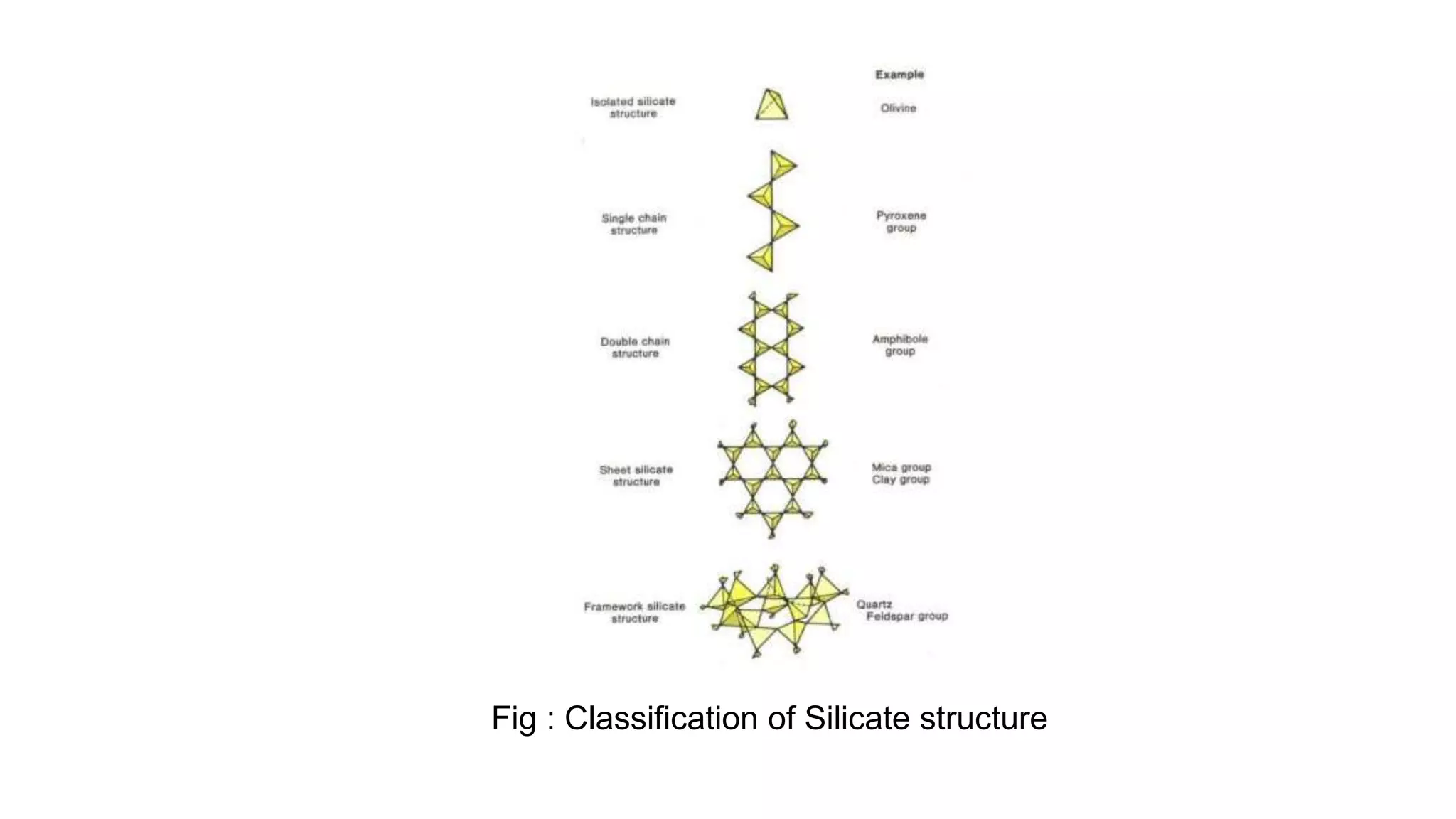
This document provides an overview of the key mineralogical properties used for mineral identification, including both chemical and physical properties. It discusses the chemical composition and classification of minerals into silicates and non-silicates. For physical properties, it describes several identification techniques such as luster, color, streak, hardness, crystal shape, cleavage, fracture and specific gravity. It also covers optical properties including color, pleochroism, habit, relief, extinction and twinning. The document is intended as a guide for mineral identification based on a comprehensive analysis of their chemical and physical characteristics.