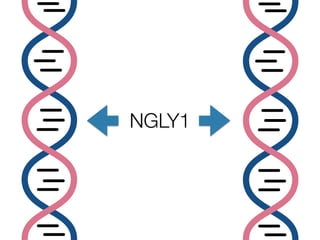
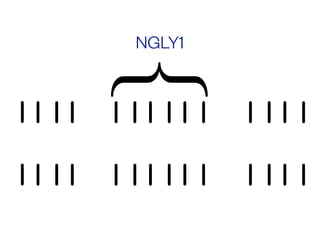
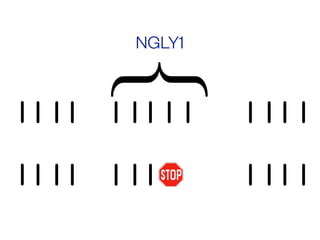
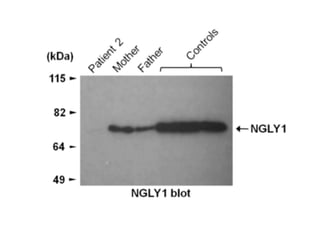
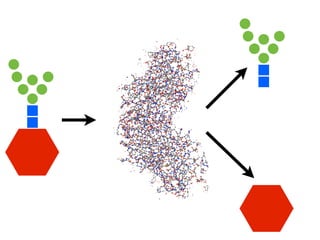
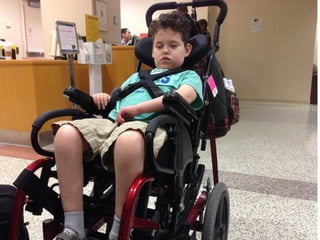
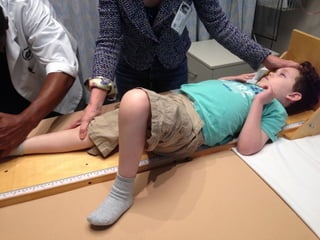
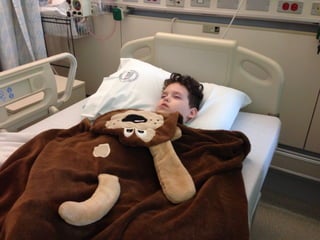
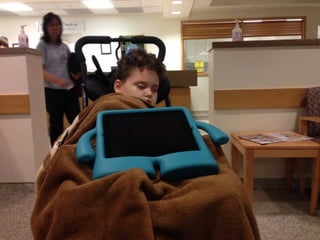
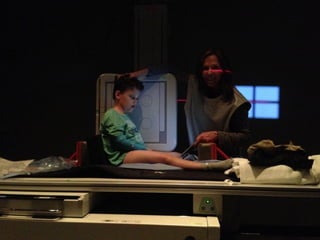
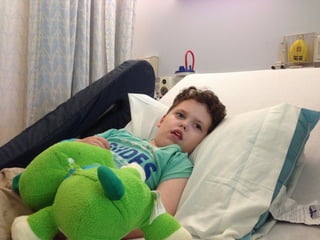
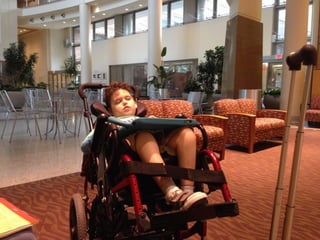
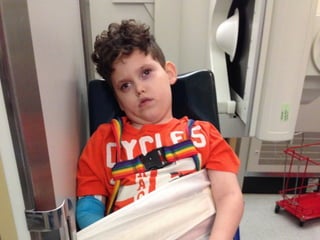
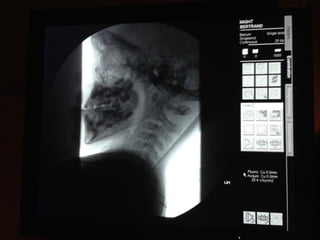
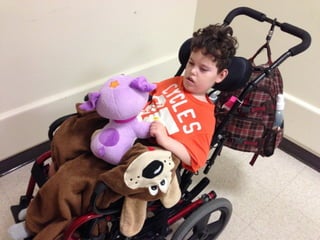
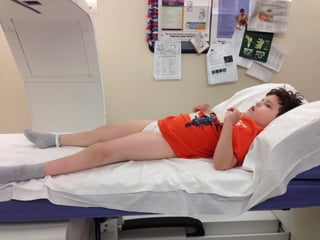
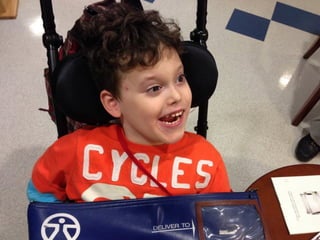
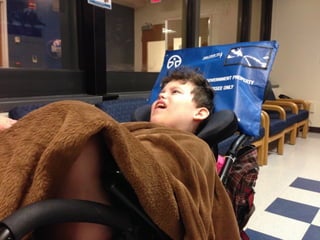
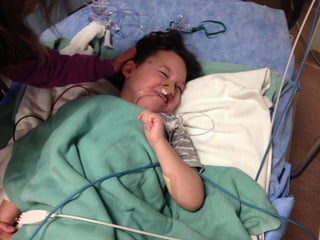
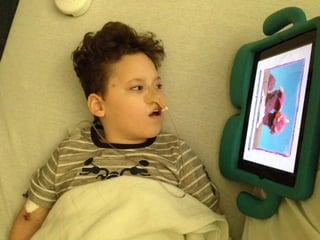
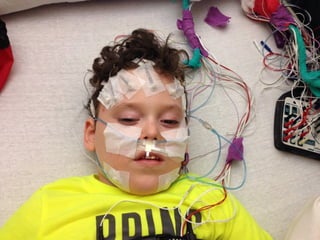
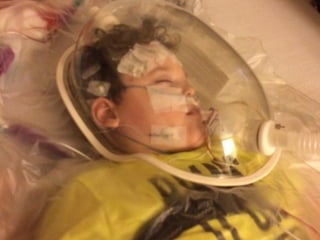
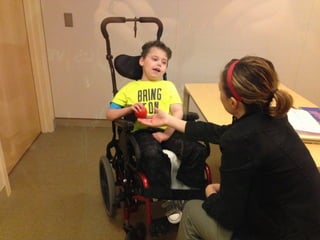
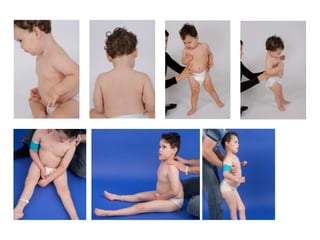
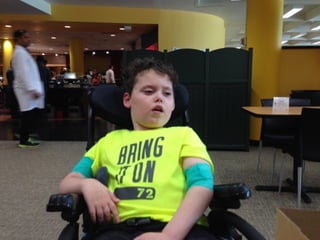
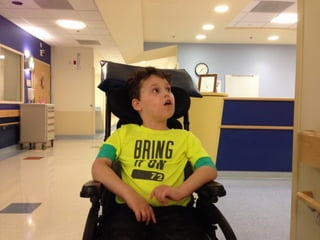
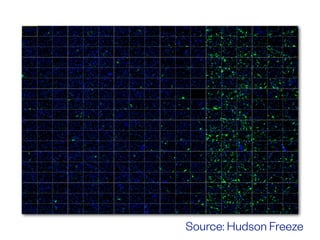
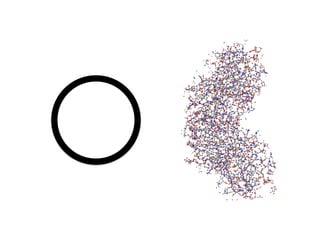
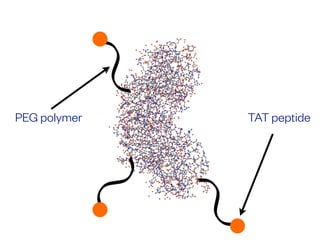
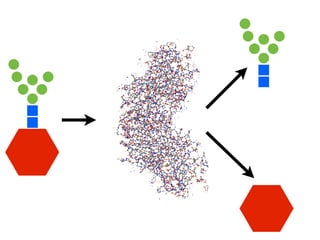
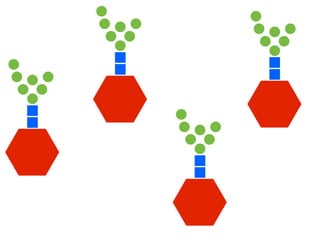
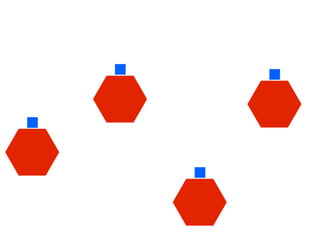
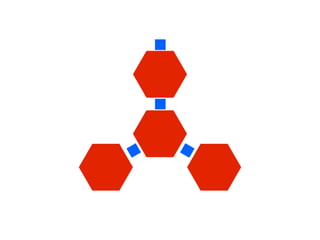
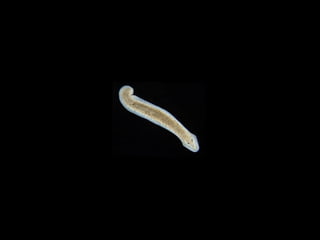
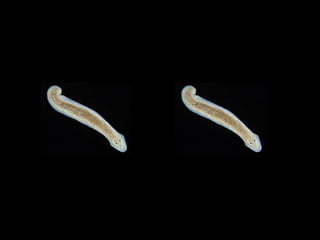
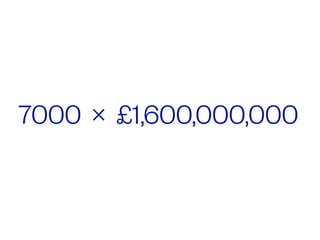The document discusses the critical role of the enzyme ngly1 in the endoplasmic reticulum-associated degradation (ERAD) process and its involvement in the pathology of a genetic disorder caused by mutations in the ngly1 gene. It highlights how the absence of ngly1 leads to dysregulation in protein degradation and the formation of protein aggregates due to alternative deglycosylation activity of another enzyme, engase. This research emphasizes the significance of n-glycans in maintaining protein quality control and could provide insights into potential therapeutic directions for patients with ngly1 deficiency.