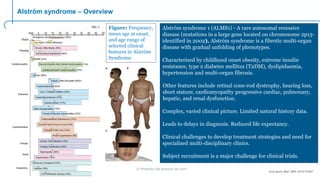
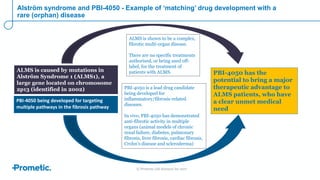
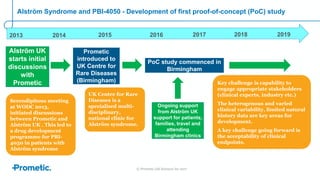
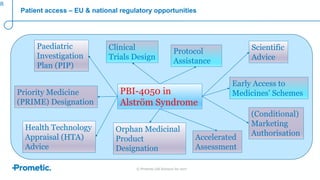
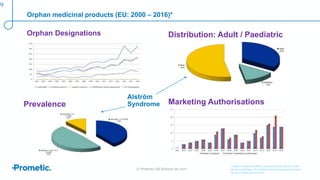
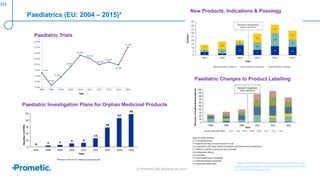
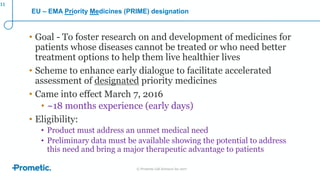
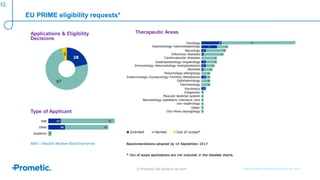
Alström syndrome is a rare genetic disease characterized by childhood obesity, insulin resistance, and fibrosis of multiple organs. There are currently no approved treatments. PBI-4050, a drug candidate from Prometic being developed for fibrosis, has potential as a novel treatment for Alström syndrome given its multi-organ anti-fibrotic activity. An ongoing proof-of-concept study of PBI-4050 in the UK represents the first clinical trial in Alström syndrome patients. Regulatory pathways like orphan drug designation and the EMA's PRIME program aim to facilitate development of treatments for rare diseases by providing scientific advice, accelerated assessment, and incentives for further research.