This document summarizes a study examining the role of the μ2 subunit of the clathrin adaptor protein AP-2 in receptor-mediated endocytosis. The researchers constructed an epitope-tagged version of μ2 that incorporated into AP-2 complexes and localized to clathrin-coated pits. Using this tagged μ2, they identified amino acids D176 and W421 as important for interacting with internalization motifs. They then generated a mutant μ2 unable to bind these motifs. Overexpression of this mutant μ2 replaced endogenous μ2 in AP-2 complexes and blocked AP-2's interaction with internalization signals. This resulted in impaired endocytosis of the transferrin receptor but not the EGF receptor, demonstrating that

![Role of the µ2 subunit of AP-2 in endocytosis
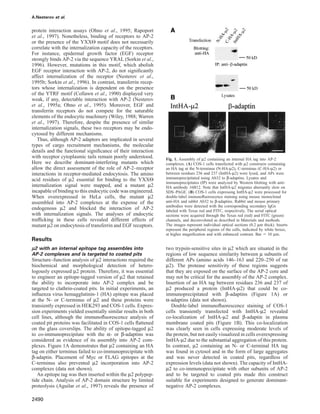
Fig. 2. Expression of epitope-tagged µ2 in HeLa cells using the tetracycline-controlled system. (A) HeLa cells expressing HA-µ2 under the
tetracycline-regulated promoter were grown for 4 days in the presence (ϩTET) or absence of tetracycline (–TET), lysed and AP-2 was precipitated
using antibody AP.6. Aliquots of cell lysates were resolved by SDS–PAGE, and expression of HA-µ2 was determined by Western blotting with anti-
HA. The immunoprecipitates were analyzed for the presence of α-adaptins (αA and αC) and µ2-proteins (endogenous and HA-tagged) by blotting
with AC1-M11 antibody and anti-µ2 serum, respectively. (B) HeLa cells expressing HA-µ2 were grown in the presence or absence of tetracycline.
Cells were permeabilized with 0.1% Triton X-100, fixed and processed for double-label immunofluorescence staining using Ab32 and anti-HA as
described in Materials and methods. Serial optical sections were acquired through the Texas Red (red) and FITC (green) channels, and deconvoluted.
The images represent individual optical sections (0.2 µm thick). The arrows indicate examples of co-localization of HA and β-adaptin staining.
Bar ϭ 5 µm.
Epitope-tagged µ2 replaces endogenous µ2 in the
AP-2 complex
To interfere efficiently with the receptor-binding function
of AP-2, heterologous µ2 should be expressed in cells at
a level high enough to substitute for its endogenous
counterpart in newly synthesized AP-2 complexes under
conditions that avoid the toxic effects of overexpression.
Inducible expression meets these requirements and, in
contrast to transient expression, allows quantitative
analysis of the cell population homogeneously expressing
a protein of interest. IntHA-µ2 (further referred to as
HA-µ2) was expressed in HeLa cells using a tetracycline-
regulated expression system (Gossen and Bujard, 1992).
Removal of tetracycline results in expression of HA-µ2
detected by anti-HA antibodies, whereas no expression
was detected in the presence of tetracycline (Figure
2A, top panel). Immunoprecipitation with the α-adaptin
antibody AP.6 followed by Western blotting with α-adaptin
antibody AC1-M11 revealed that the number of AP-2
complexes was not changed, regardless of the presence
of tetracycline (Figure 2A, middle panel). HA-µ2, which
can be distinguished from endogenous µ2 by a slower
electrophoretic mobility, co-immunoprecipitates with
α-adaptins as shown by blotting with the antibody to µ2,
confirming the assembly of HA-tagged µ2 into the AP-2
complex (Figure 2A, bottom panel).
These experiments also demonstrate the ability of
epitope-tagged µ2 to substitute for endogenous µ2 in AP-2
complexes. Since the half-life of AP-2 is ~24 h (Sorkin
et al., 1995), the complete turnover of the AP-2 pool was
expected to take several days. After 4 days of expression,
HA-µ2 represented at least 80–90% of the total amount of
µ2 protein assembled into AP-2 (Figure 2A). Interestingly,
analysis of cellular lysates revealed that overexpression
of HA-µ2 caused almost complete disappearance of endo-
genous µ2 (data not shown). This observation suggests
2491
the existence of mechanisms that eliminate excessive µ2
which is not assembled into AP-2, and may serve at a post-
translational level to control the stoichiometry between
subunits of the AP-2 complex.
The localization of HA-µ2 in HeLa cells was examined
by double-label immunofluorescence staining after cell
permeabilization with Triton X-100. Figure 2B shows the
appearance of punctate staining with anti-HA in the
absence of tetracycline and the co-localization of HA-µ2
staining with β-adaptins, indicating that AP-2 containing
HA-µ2 is targeted correctly to plasma membrane and
allows formation of coated pits.
Point mutations D176A and W421A disrupt the
interaction of µ2 with internalization signals
without interfering with incorporation into AP-2
To identify residues in µ2 that are essential for the
interaction with YXXΘ internalization signals, several
µ2 mutants were generated. The wild-type or mutant µ2
constructs were either translated in vitro in the presence
of [35S]methionine (Figure 3A) or transiently expressed
in COS-1 or HEK293 cells (Figure 3B). The µ2 proteins
were tested for their ability to interact with GST fused
either with triple repeats of the internalization signal
SDYQRL from the transmembrane protein TGN38 (GST–
TGN38) or with the residues 908–1186 of the C-terminus
of the EGF receptor (GST–EGFR). To control for non-
specific interactions, GST alone or a GST–EGFR mutant,
whose AP-binding sequence Y974RAL (Sorkin et al., 1996)
was mutated to ARAL (GST–EGFRY974A), were used.
Both GST–TGN38 and GST–EGFR were equally potent in
binding in vitro translated µ2. GST–EGFR also efficiently
precipitated cellular AP-2 complexes containing transiently
expressed HA-µ2. In contrast, we have not been able to
detect the specific binding of cellular AP-2 to GST–
TGN38.](https://image.slidesharecdn.com/f3e1319c-ecbc-49dc-b4f2-a5ade7337b36-160118185808/85/EMBO-3-320.jpg)
![A.Nesterov et al.
Fig. 3. Effects of point mutations in µ2 on interactions with TGN38
and EGF receptor. (A) Fragments of µ2 (∆120µ2, residues 121–435)
were translated in the presence of [35S]methionine using the TNT
system. Where indicated, residues D176, D269 or M209 of µ2 were
changed to alanine. In vitro translated µ2 fragments were incubated
with glutathione–agarose beads containing either GST–TGN38 or GST
alone. Precipitates were analyzed by electrophoresis followed by
fluorography. (B) COS-1 cells transfected with the wild-type or
D176A mutant HA-µ2 were lysed, and the lysates were incubated with
glutathione–agarose beads containing GST–EGFR or GST alone. The
agarose precipitates and aliquots of lysates were analyzed by Western
blotting with anti-HA.
It has been reported that mutation of µ2 residues D176,
M209 or F265 to alanine inhibits the interaction of µ2
with the TGN38 internalization signal in yeast two-hybrid
assays (Aguilar et al., 1997). Similar mutations were made
in µ2 lacking the first 120 N-terminal residues and
the mutants were expressed in reticulocyte lysates. The
N-terminal truncated µ2 was used because deletion of the
120 residues of the N-terminus of µ2 has been shown to
increase the affinity of µ2 binding to TGN38 in yeast
two-hybrid and in vitro pull-down assays (Ohno et al.,
1995, 1996). In GST pull-down experiments with in vitro
translated µ2, only the D176A mutation completely abol-
ished the µ2 interaction with TGN38, whereas the M209A
mutation resulted in a partial inhibition (Figure 3A,
upper panel). Similar results were obtained in pull-down
experiments with GST–EGFR (data not shown). Surpris-
ingly, binding of µ2 transiently expressed in COS-1 cells
to GST–EGFR was only moderately affected by D176A
mutation (Figure 3B).
To generate mutations in µ2 that would inhibit inter-
action with both TGN38 and EGF receptor internalization
signals, several µ2 mutants with C-terminal truncations
were prepared. GST pull-down experiments revealed that
whereas µ2 truncated at residue 428 (CЈ428) partially
retained the ability to bind GST–EGFR and GST–TGN38,
µ2 truncated at residues 420 (CЈ420) and 409 (CЈ409)
(Figure 4A and B) did not bind internalization signals.
Therefore, the sequence between residues 420 and 428 is
2492
important for HA-µ2 interaction with YXXΘ motifs.
However, truncated µ2 mutants were not useful because
these mutants were not incorporated into AP-2 (Figure
5A). The observation that modifications of the C-terminus
of µ2 by small deletions or extensions with epitope
tags (Figures 1 and 5) abrogate µ2 co-precipitation with
adaptins implicates this region in AP-2 assembly.
The region 420–428 contains the sequence WVRYI that
is conserved in µ2 from yeast to mammals and in the µ1
subunit of AP-1. This region, and particularly amino acid
residue W421, could be part of a hydrophobic pocket
involved in the interaction with either the first tyrosine or
the last hydrophobic residue of the internalization signal.
Consistent with this hypothesis, the substitution of W421
by alanine (W421A) completely abolished the interaction
of HA-µ2 with both GST–TGN38 (Figure 4B) and GST–
EGFR (Figure 4C). The interaction of wild-type µ2 with
GST–EGFR required Tyr974 of the EGF receptor (Figure
4C). Thus, mutagenesis and GST pull-down assays
revealed that at least two regions of the µ2 molecule
containing residues D176 and W421 are engaged in µ2
interaction with YXXΘ motifs. That D176A and W421
are directly involved in the interaction with internalization
signal peptides has now been demonstrated by the crystal
structure data published during the preparation of this
manuscript (Owen and Evans, 1998).
To inhibit maximally the effects the receptor-binding
function of µ2, both D176 and W421 residues of µ2 were
changed to alanines. The double mutant was expressed in
HeLa cells using a tetracycline-regulated system, and
interaction of the mutant µ2 protein with GST–EGFR was
examined. As shown in Figure 4D, the D176A/W421A
HA-µ2 was severely impaired in its ability to bind
GST–EGFR.
In contrast to truncations, the point mutations D176A
and W421A did not affect the ability of µ2 to co-
immunoprecipitate with α-adaptins (Figure 5A). Figure 5B
demonstrates that the double mutant D176A/W421A
expressed in HeLa cells was also immunoprecipitated with
α-adaptins. As demonstrated for wild-type HA-µ2 (Figure
2) when the expression of D176A/W421A µ2 was induced
by tetracycline withdrawal, the mutant displaced endo-
genous µ2 from the cellular AP-2 complexes (Figure 5B).
Inducible overexpression of the D176A/W421A µ2
mutant inhibits interaction of AP-2 with the EGF
receptor
To investigate how the displacement of endogenous µ2
by the mutant µ2 affects interaction between the whole
AP-2 complex and YXXΘ signals, lysates from HeLa
cells expressing either wild-type or D176A/W421A µ2
were incubated with GST–EGFR. The amount of bound
AP-2 was determined by the presence of α-adaptin in
GST precipitates. It was found that AP-2 obtained from
cells expressing wild-type HA-µ2 bound very efficiently
to GST–EGFR and did not bind to either the GST–
EGFRY974A mutant or GST alone (Figure 6A). In
contrast, when expression of HA-µ2-D176A/W421A was
induced by the removal of tetracycline, binding of AP-2
to GST–EGFR was abolished completely (Figure 6B).
To confirm that the inhibition was specific to the
plasma membrane adaptor AP-2, the effect of mutant µ2
overexpression on the interaction between GST–EGFR](https://image.slidesharecdn.com/f3e1319c-ecbc-49dc-b4f2-a5ade7337b36-160118185808/85/EMBO-4-320.jpg)

![A.Nesterov et al.
Fig. 6. D176A/W421A µ2 mutant inhibits interaction of AP-2 with the
EGF receptor C-terminus. GST–EGFR, GST–EGFRY974A or GST
alone were bound to glutathione–agarose beads, and incubated with
lysates from HeLa cells expressing (–TET) or not expressing (ϩTET)
either wild-type HA-µ2 (A) or D176A/W421A HA-µ2 (B). The
agarose precipitates were resolved through SDS–PAGE and the
amounts of bound AP-2 and AP-1 were determined by Western
blotting with antibodies AC1-M11 to α-adaptins or 100/3 to γ-adaptin.
Inhibition of the interaction between AP-2 and
tyrosine-based internalization signals produces
contrasting effects on endocytosis of different
receptors
To investigate how inhibition of the AP-2 receptor-binding
function affects receptor-mediated endocytosis, we
analyzed the effect of dominant-negative µ2 on the
internalization kinetics of EGF and transferrin receptors.
Both receptors are naturally expressed in HeLa cells and
contain tyrosine-based internalization signals with the AP-
binding consensus sequences (Collawn et al., 1990; Chang
et al., 1993a). Three cell lines, inducibly expressing wild-
type, single mutant D176A µ2 and double mutant D176A/
W421A µ2, were examined for the rates of internalization
of labeled transferrin and EGF. As shown in Figure 7,
[125I]transferrin was internalized rapidly in all cell lines
grown in the medium containing tetracycline. However,
induction of the expression of D176/W421 µ2 produced
a 4-fold decrease in the rate of internalization of transferrin,
whereas this rate was unaffected by the expression of
wild-type HA-µ2. Moreover, a continuous inhibition of
2494
Fig. 7. Internalization of [125I]EGF and [125I]transferrin in HeLa cells.
HeLa cells expressing wild-type HA-µ2 (WT, circles), HA-D176A µ2
(D716A, squares) or HA-D176A/W421A µ2 (D176A/W421A,
triangles) mutants were grown in the presence (ϩTet) or absence of
tetracycline (–Tet). Cells were incubated with 1 µg/ml [125I]transferrin
or 1 ng/ml [125I]EGF for 2–6 min and the amount of surface-bound
and internalized radioactivity was determined as described in Materials
and methods. The rate of internalization is expressed as the ratio of
internalized and surface [125I]ligand for each time point. (A) Time
course of transferrin or EGF internalization. (B) Internalization rates
averaged from several experiments in cells grown without tetracycline.
Four single cell clones expressing the D176A/W421A mutant were
examined. The rates are expressed as a percentage of these rates in
cells grown with tetracycline where expression of HA-µ2 proteins is
inhibited. (C) Time course of [125I]EGF internalization in control and
Kϩ-depleted (–Kϩ) cells. The results are representative of several
experiments with two clones of cells expressing the D176A/W421A
µ2 mutant.
transferrin receptor endocytosis as a result of the expres-
sion of the D176A/W421A mutant resulted in a 4-fold
increase in the surface pool of these receptors (data
not shown). The single mutant D176A µ2 imposed an
intermediate effect on transferrin endocytosis (Figure 7).
These data indicate that the internalization of the transferrin
receptor requires interaction of the receptor with the µ2
subunit of AP-2.
In parallel experiments, the internalization rates of
[125I]EGF were measured. As with the transferrin receptor,
the internalization parameters of EGF were similar in
different cell clones in the presence of tetracycline (Figure
7). However, in contrast to transferrin endocytosis,
internalization of EGF was not significantly affected by
overexpression of D176/W421 µ2 (Figure 7). Furthermore,
the extent and the rate of EGF-induced down-regulation
of EGF receptors was independent of the expression of
the wild-type or mutant µ2 (data not shown).](https://image.slidesharecdn.com/f3e1319c-ecbc-49dc-b4f2-a5ade7337b36-160118185808/85/EMBO-6-320.jpg)
![Role of the µ2 subunit of AP-2 in endocytosis
To test whether the internalization of EGF receptors in
cells expressing mutant µ2 is clathrin dependent, the effect
of Kϩ depletion on [125I]EGF uptake was examined. Kϩ
depletion of the cells is known to block the assembly of
coated pits and clathrin-mediated endocytosis (Larkin
et al., 1983). As shown in Figure 7C, Kϩ depletion
abolished EGF internalization in cells grown in the pres-
ence or absence of tetracycline. These data suggest that
endocytosis of the EGF receptor is mediated by clathrin-
coated vesicles, but does not require the interaction of the
receptor with µ2.
Discussion
We applied a dominant-interfering approach to investigate
the functional significance of the interaction between
the tyrosine-based internalization YXXΘ signal and the
plasma membrane adaptor protein AP-2. Since in vitro
interactions between YXXΘ motifs and AP-2 were shown
to be mediated by µ2, we generated a mutant of µ2 that
does not bind internalization sequences, but is capable of
substituting for the endogenous µ2 in AP-2 complexes.
The expression of such a mutant was expected to inhibit
the interaction of receptor cytoplasmic tails with AP-2,
and thus could be used to assess the importance of this
interaction for endocytosis of different receptors.
Generation of suitable µ2 mutants first required con-
struction of an epitope-tagged version of µ2 that assembles
into AP-2 and does not interfere with the intracellular
targeting of AP-2. Placing a small HA sequence at
the N-terminus of µ2 inhibited incorporation into AP-2
complexes. This result is consistent with the direct inter-
action of the N-terminus of µ2 with β-adaptin demonstrated
in two-hybrid assays (Aguilar et al., 1997). Unexpectedly,
µ2 proteins containing small epitope tags at the C-terminus
or missing only the last seven amino acid residues were
unable to associate with adaptins, indicating that the
C-terminus of µ2 is also important for the AP-2 complex
assembly. Since this region of µ2 did not appear to be
important for binding to β-adaptins, it may be involved
in the interaction with other AP-2 subunits or required for
the proper folding of the µ2 protein. Insertion of the HA
epitope into an internal region of low sequence similarity
among adaptor medium chains did not prevent µ2 assembly
into AP-2 or its targeting to coated pits. An antibody to
the epitope tag recognized AP-incorporated µ2 under
non-denatured conditions in immunoprecipitation and
immunofluorescence experiments. Together with data
obtained by limited proteolysis (Aguilar et al., 1997),
these results suggest that the region encompassing residues
236 and 237 is exposed on the surface of the AP-2 core.
Several residues important for the binding of µ2 to
the YQRL internalization signal in TGN38 have been
identified previously in yeast two-hybrid assays (Aguilar
et al., 1997). In GST pull-down experiments, however,
only mutation D176A significantly affected µ2 interaction.
The reason for this discrepancy is presently unclear, and
may be related to the differences in folding of µ2 proteins
expressed in yeast, translated in vitro or expressed in
mammalian cells. Interestingly, whereas the D176A muta-
tion strongly inhibited µ2 binding to the YQRL motif of
TGN38, the interaction of this mutant with the YRAL
sequence of the EGF receptor was only partially affected.
2495
Further mapping of the receptor-binding sequences
revealed that mutation of the conserved W421 yielded µ2
protein defective in binding to both the EGF receptor and
TGN38. To suppress completely the interaction of µ2 with
several types of YXXΘ consensus internalization signals,
the double mutant D176A/W421A was used in expres-
sion studies.
During the preparation of this manuscript, the crystal
structure of the part of the µ2 protein (residues 158–
435) complexed with tyrosine-containing peptides was
published (Owen and Evans, 1998). Interestingly, both
function-suppressive mutations that we identified involve
residues that are located within the tyrosine peptide-
binding pocket and directly participate in the interaction
with this peptide. The crystal structure demonstrates
multiple interactions within the binding pocket, possibly
explaining why the mutation D176A had only partial
effects on the EGF receptor interaction with µ2. Appar-
ently, in the absence of hydrogen bonds between the
hydroxyl group of Y974 of the EGF receptor and D176
of µ2, the hydrophobic interactions of Y974 with W421
and F174 of µ2 as well as that of L977 of the EGF
receptor with several aliphatic residues within the binding
pocket of µ2 are sufficient to support the complex. The
structure also revealed that the site of insertion of the HA
tag lies within the unfolded loop between the β3- and β4-
strands of µ2, which explains why this modification did
not affect µ2 functions.
Thus, independently of the structural data, mutational
analysis identified residues D176 and W421 as a part of
the internalization signal-binding interface of µ2 and, in
addition, demonstrated that this binding pocket is the
binding site in the holo µ2 protein. Another YXXΘ-
binding interface was proposed to exist within the N-
terminal domain of µ2 (residues 102–125) (Bremnes
et al., 1998) that is situated outside of the structurally
characterized part of the protein. However, as the D176A/
W421Aµ2 mutations completely abrogated the binding
abilities of full-length µ2, region 102–125 does not appear
to be essential for the interaction with YXXΘ motifs.
Several approaches to achieve a maximal dominant-
negative effect of mutant µ2 were tested. Transient expres-
sion resulted in accumulation of a large fraction of
µ2 protein in cytosolic aggregates and did not allow
quantitative measurements of endocytosis. The selection
of constitutively expressing stable cell lines yielded clones
containing low levels of exogenous protein. The tetracyc-
line-controlled expression system proved to be the most
suitable: it generated high expression levels sufficient to
substitute metabolically for endogenous µ2 in the majority
of cellular AP-2 complexes in all cells in the population,
making possible quantitative measurements of endocytic
kinetics. Importantly, the generation of dominant-negative
AP-2 incapable of binding to YXXΘ motifs did not
increase the total amount of AP-2 in the cell. Thus, in
contrast to dominant-negative approaches that involve
simple overexpression of a protein or a fragment of a
protein, this strategy allowed investigation of functions
for a particular type of adaptor protein without interfering
with other APs. AP-2 complexes containing mutant µ2
were properly targeted to the plasma membrane, and in
general behaved indistinguishably from control AP-2 (data
not shown), indicating that the interaction of the cyto-](https://image.slidesharecdn.com/f3e1319c-ecbc-49dc-b4f2-a5ade7337b36-160118185808/85/EMBO-7-320.jpg)
![A.Nesterov et al.
plasmic tails with AP-2 via µ2 does not play a significant
role in AP-2 targeting and docking to the membrane.
To investigate how inhibition of the signal recognition
function of AP-2 affects receptor-mediated endocytosis,
we measured the endocytic kinetics of EGF and transferrin
receptors in cells expressing a dominant-interfering mutant
of µ2. Both receptors are internalized via clathrin-coated
pits in HeLa cells, and contain YRAL and YTRF
sequences, respectively, which are implicated in internaliz-
ation (Collawn et al., 1990; Chang et al., 1993b; Sorkin
et al., 1996). Mutational inactivation of the YTRF motif
of the transferrin receptor abolished endocytosis (Collawn
et al., 1990). Despite the apparent low affinity interaction
between the transferrin receptor and AP-2 (Nesterov et al.,
1995a; Ohno et al., 1995) that, to our knowledge, has not
been confirmed by co-immunoprecipitation, the experi-
ments in cells expressing mutant µ2 directly demonstrate
that the interaction of the transferrin receptor with the µ2
subunit of AP-2 is essential for the receptor endocytosis.
Studies of EGF receptor endocytosis have suggested
several mechanisms. EGF receptors can be co-immuno-
precipitated with AP-2, bind AP-2 in vitro and interact
with the µ2 subunit in yeast two-hybrid assays (Sorkin
and Carpenter, 1993; Boll et al., 1995; Nesterov et al.,
1995a; Sorkina et al., 1999). The strong EGF receptor
interaction with AP-2 is mediated by the Y974RAL
sequence of the receptor (Sorkin et al., 1996). However,
mutations in this sequence had no effect on EGF receptor
endocytosis in cells expressing physiological levels of
receptors (Nesterov et al., 1995b; Sorkin et al., 1996).
Based on these observations, it was concluded that the
high affinity interaction of the EGF receptor with AP-2
was not important for endocytosis. However, the possibility
that EGF receptors associate with AP-2 via other weak
binding sites or through the formation of heterodimers
with other members of the Erb receptor family (Gilboa
et al., 1995; Lenferink et al., 1998) could not be excluded.
The present experiments with a dominant-negative mutant
of µ2 demonstrate that the interaction of µ2 with EGF
receptors is not essential for receptor internalization, and
support the notion that alternative mechanisms control the
endocytosis of this receptor (Lamaze et al., 1993). These
mechanisms may involve newly characterized clathrin-
binding molecules such as β-arrestins (Ferguson et al.,
1996) participating in the recruitment of other classes of
receptors into coated pits or the terminal domains of
clathrin heavy chain (Kibbey et al., 1998). A number of
proteins containing SH2 or PTB domains that are capable
of binding to the activated EGF receptor may also play a
role in the targeting of the EGF receptors to coated pits.
What is the functional role of EGF receptor interaction
with AP-2? It is possible that this interaction has a
role in the internalization of EGF receptors in cells
overexpressing these receptors. The highest extent of EGF
receptor association with AP-2 was detected in A-431
cells which display an extraordinary high density of
EGF receptors (Sorkin and Carpenter, 1993). Moreover,
mutations of the Y974-containing motif of the receptor
resulted in impaired endocytosis in NIH 3T3 cells
expressing high levels of receptors (Sorkin et al., 1996).
The results presented in this study thus demonstrate
that endocytosis may proceed through both µ2-dependent
and -independent mechanisms. The data also illustrate
2496
the usefulness of a dominant-interfering approach to
investigate functions of various internalization signals and,
potentially, other adaptor protein complexes.
Materials and methods
Reagents
Pfu polymerase, Taq precision polymerase and the QuickChange site-
directed mutagenesis kit were from Strategene Cloning Systems (La Jolla,
CA). The in vitro coupled transcription and translation system (TNT) was
from Promega Corporation (Madison, WI). The mammalian expression
vector pcDNA3 was from Invitrogen Corp. (Carlsbad, CA) and the
tetracycline-controlled expression vector pUHG 10-3 was a gift of
H.Bujard (ZMBH, Heidelberg, Germany). The bacterial expression
vector pGEX-4T3 was purchased from Amersham Pharmacia Biotech
(Piscataway, NJ), B-PER reagent from Pierce (Rockford, IL), tetracycline
and puromycin from Calbiochem-Novabiochem Corporation (La Jolla,
CA), and sodium butyrate and glutathione–agarose beads from Sigma
(St Louis, MO).
Iron-saturated human transferrin was purchased from Sigma and
iodinated using Iodo-Beads (Pierce, Inc.). Mouse receptor-grade EGF
was obtained from Collaborative Research Inc. and iodinated using a
modified chloramine-T method as described previously (Carpenter and
Cohen, 1976). The specific activity of [125I]transferrin and [125I]EGF
was 3.0ϫ105 c.p.m./µg and 1.5–1.9ϫ105 c.p.m./ng, respectively.
Antibodies
Monoclonal antibody to the α-subunit of AP-2 (AP.6) (Chin et al., 1989)
was obtained from ATCC, whereas the monoclonal antibody 100/3
specific to γ-adaptin was from Sigma. Monoclonal AC1-M11 antibody
to α-adaptin and polyclonal antiserum specific to the µ2 subunit
were provided by M.Robinson (Cambridge University, UK). AC1-M11
antibody was also purchased from Affinity Bioreagents Inc. (Golden,
CO). Rabbit polyclonal antibody Ab32 specific to β-adaptins was
described previously (Sorkin et al., 1996). Monoclonal antibody 16B12
to the HA epitope tag was purchased from BABCO (Richmond, CA).
Plasmid constructs
cDNA encoding rat µ2 (Thurieau et al., 1988) provided by T.Kirchhausen
(Harvard University, Boston, MA) was amplified using Pfu polymerase
and subcloned either into the constitutive expression vector pcDNA3 or
into the tetracycline-controlled expression vector pUHG 10-3 (Gossen
and Bujard, 1992) provided by H.Bujard (ZMBH, Heidelberg, Germany).
In order to generate µ2 containing a C-terminal HA-1 epitope tag,
the sequence encoding YPYDVPDYA was introduced into the 3Ј
amplification oligonucleotide. µ2 containing an internal HA epitope was
generated by a two-step amplification procedure, which introduced the
sequence YPYDVPDYALE between residues 236 and 237. To place an
HA tag at the N-terminus, µ2 cDNA encoding residues 2–435 was
subcloned in-frame to a modified pcDNA3 plasmid generating
an N-terminal extension MEYPYDVPDYAEFCRYPCHWRPLE.
N-terminal truncation mutant N-120 and C-terminal truncation mutants
CЈ409, CЈ420 and CЈ428 were generated by amplification of µ2 cDNA
using Pfu polymerase. All point mutations in µ2 constructs were
generated using the QuickChange site-directed mutagenesis kit according
to the manufacturer’s protocol.
A fragment of EGF receptor corresponding to the C-terminus of the
receptor (amino acids 908–1186) was amplified using Taq precision
polymerase and subcloned into the bacterial expression vector pGEX-
4T3 to yield GST–EGFR. A tyrosine to alanine mutation of the residue
equivalent to Tyr974 in the holo-EGF receptor was introduced into
GST–EGFR using the QuickChange method to yield GST–EGFRY974A.
Synthetic oligonucleotides encoding a triple repeat of the internalization
signal of TGN38 (SDYQRLSDYQLLSDYQRLNLKL) were annealed
and cloned in-frame in the GST expression vector pGX-KG to yield
GST–TGN38.
All constructs were verified by dideoxynucleotide sequencing. The
sequences of oligonucleotides used for cloning are available upon request.
Expression of GST fusion proteins
GST–TGN38 was made in Escherichia coli BL21 strain. Expression of
proteins was induced by 0.2 mM isopropyl-β-D-thiogalactopyranoside
(IPTG) for 5 h, cells were collected by centrifugation, washed in ice-
cold 100 mM NaCl, supplemented with 20 mM Tris–HCl pH 8.0 and
frozen. The cell pellet was resuspended in B-PER reagent containing](https://image.slidesharecdn.com/f3e1319c-ecbc-49dc-b4f2-a5ade7337b36-160118185808/85/EMBO-8-320.jpg)
![Role of the µ2 subunit of AP-2 in endocytosis
protease inhibitors (5 ml of B-PER per 1 l of bacterial culture), sonicated
and cleared by centrifugation. Aliquots of bacterial lysate were stored
at –80°C.
GST–EGFR and GST–EGFRY974A were made in E.coli B834 (DE3)
pLysS, which were grown in LB (supplemented with ampicillin and
chloramphenicol) to A260 ~0.6. The expression was induced with 0.1 mM
IPTG for 2–3 h at 37°C. The cells were harvested and washed once in
50 mM Tris, 2 mM EDTA, 1 mM phenylmethylsulfonyl fluoride (PMSF)
pH 8.0 and frozen at –80°C. The cell pellets were sonicated in 50 mM Tris,
4 mM EDTA, 2 mM EGTA, 1 mM dithiothreitol (DTT), 25% (w/v) sucrose
pH 8.0supplemented with1% Triton X-100,1% proteasecocktail (Sigma),
10 mg/ml aprotinin, 10 mg/ml leupeptin and 10 mg/ml iodacetamide. After
20 min centrifugation at 100 000 g, the supernatanats were bound to
glutathione–Sepharose 4B beads (Pharmacia) at the ratio of 700 ml per
50 ml of starting culture for 3 h at 4°C. The beads were washed three
times in ice-cold Ca2ϩ, Mg2ϩ-free phosphate-buffered saline (CMF-PBS),
resuspended in an equal volume of fresh CMF-PBS to give a 50% (v/v)
slurry, and stored at 4°C.
Mammalian cell culture and transfections
HEK293 cells were grown in Dulbecco’s modified Eagle’s medium
(DMEM) containing 10% fetal bovine serum (FBS), antibiotics and
glutamine. COS-1 cells were grown in DMEM containing 10% newborn
calf serum, antibiotics and glutamine. HeLa cells expressing tet-off
transactivator (Damke et al., 1995) were provided by S.Schmid (Scripps
Institute, La Jolla, CA). HeLa cells were grown in DMEM containing
10% FBS, antibiotics and glutamine, and supplemented with G418.
For transient expression, cells grown to 50–80% confluencey were
transfected with pcDNA3 plasmids using a calcium phosphate transfec-
tion kit (5Ј–3Ј, Boulder, CO), Lipofectamine (Gibco-BRL) or Effectene
(Qiagen). Transient transfections were used for all experiments with
COS-1 and HEK293 cells. Transfected cells were split 1 day after
transfection to dishes and glass coverslips and used for experiments on
the second or third day. Cells were grown to ~90 or 50% confluency
for co-immunoprecipitation or immunofluorescence experiments,
respectively.
HeLa cell lines expressing µ2 constructs under the tetracycline-off-
controlled promoter were generated as outlined in Damke et al. (1995).
The cells were grown in DMEM supplemented with 10% fetal calf
serum, 400 µg/ml G418, 100 U/ml penicillin, 100 U/ml streptomycin
and 0.25 µg/ml amphotericin B. Cells (5ϫ105) were transfected using
the calcium phosphate method with 17 µg of expression vector pUHG10-3
containing various µ2 constructs mixed with 1.5 µg of plasmid pBSpac,
containing the puromycin resistance selection marker. Individual colonies
selected in the presence of 200 ng/ml puromycin and 2 µg/ml tetracycline
were isolated and analyzed for the expression of epitope-tagged µ2
constructs by Western blotting using antibody 16B12.
For the experiments, cells were plated in the growth medium that did
not contain selection markers with or without tetracycline. At 24 h after
plating, medium was replaced with the fresh medium with or without
tetracycline supplemented with 2 mM sodium butyrate to ensure the
high rates of HA-µ2 expression necessary to replace endogenous
µ2 from AP-2 complexes. Experiments were performed 3–4 days
after plating.
GST pull-down experiments
COS-1, HEK293 or HeLa cells expressing µ2 proteins were washed
with CMS-PBS and lysed in Triton/glycerol/HEPES buffer (TGH):
50 mM HEPES pH 7.4, 1% Triton X-100, 10% glycerol, 100 mM NaCl,
1 mM NaF, 1 mM EDTA, 1 mM EGTA, 1 mM PMSF, 10 mg/ml
leupeptin, 10 mg/ml aprotinin, 10 mM benzamidine for 10 min at 4°C.
The lysates were then cleared by centrifugation for 45 min at 125 000 g
in a Beckman TLA 45 rotor.
35S-Labeled µ2 proteins were made by in vitro transcription and
translation from a T7 promoter using the Promega TNT kit, according
to the supplier’s protocol. Prior to pull-down experiments, aliquots of
TNT reactions (10% of the total reaction mixure) were electrophoresed
and the amounts of labeled µ2 proteins were determined by phosphor-
imaging. Typically, these amounts varied by not more than 15%. Between
43 and 47 µl of TNT reactions corresponding to equivalent amounts of
the labeled proteins were diluted with 1 ml of TGH containing 25 mg/ml
bovine serum albumin (BSA) and cleared by centrifugation for 45 min
at 125 000 g.
For GST–TGN38 binding experiments, aliquots (0.5 ml) of E.coli
lysates were incubated with 10 µl of glutathione–agarose beads for 2–3 h
at 4°C. The beads were then washed once with 1 ml of TGH containing
25 mg/ml BSA, and incubated with 0.2–0.5 ml of TGH-diluted TNT
2497
reactions for 2–5 h at 4°C, washed three times with TGH and heated in
30 µl of SDS sample buffer. The precipitates from the TNT reactions
were separated by SDS–PAGE, gels were dried and exposed to X-ray
films and analyzed by phosphorimaging. The pull-down experiments
with GST–EGFR and in vitro translated µ2 were carried out similarly.
However, because of the low yield of GST–EGFR protein, the ratio of
specific and non-specific signals in these experiments was lower than
that in experiments with GST–TGN38.
In binding experiments with HA-µ2 expressed in vivo, beads loaded
with GST, GST–EGFR and GST–EGFRY974A were washed once in
CMF-PBS and incubated with mammalian cell lysates for 3 h at 4°C.
The beads were washed three times with 600 ml of 150 mM KCl,
20 mM HEPES, 2 mM MgCl2 pH 7.2, then heated in sample buffer for
5 min at 80°C. The precipitates from cell lysates were then resolved by
SDS–PAGE, and the proteins were transferred to nitrocellulose mem-
branes and probed by Western blotting with various antibodies to AP-2
subunits or to the HA epitope tag followed by species-specific secondary
antibodies or protein A (Zymed, Inc.) conjugated with horseradish
peroxidase. The enhanced chemiluminescence kits were from Pierce and
Amersham.
Immunoprecipitation of APs
TGH lysates were prepared from µ2-expressing cells, and cleared as
described above. APs were immunoprecipitated with AP.6 or Ab32
antibodies specific to α- or β-adaptins, respectively. The precipitates
were washed with TGH, denatured by heating in sample buffer, and
resolved on SDS–PAGE followed by transfer to the membrane and
Western blotting with various antibodies to AP subunits and anti-HA.
Immunofluorescence staining
COS-1 cells transiently expressing µ2 proteins were grown on coverslips.
The cells were washed with CMF-PBS and fixed with freshly prepared
4% paraformaldehyde (Electron Microscopy Sciences) for 12 min at
room temperature, and mildly permeabilized using a 3 min incubation
in CMF-PBS containing 0.1% Triton X-100 and 1% BSA at room
temperature. HeLa cells expressing µ2 proteins under the control of the
tetracycline-dependent promoter were permeabilized mildly with 0.1%
Triton X-100 for 3 min at 4°C before fixation. This pre-treatment
eliminated a significant background staining of HeLa cells with anti-HA
antibodies but does not affect staining of clathrin-coated pits with various
antibodies to clathrin and AP-2. We found that monoclonal 16B12
antibody is the only anti-HA antibody that recognizes the internal HA
tag. Therefore, for double-labeling, this monoclonal antibody was paired
with a polyclonal antibody to β-adaptin, Ab32.
Fixed cells were incubated in CMF-PBS containing 1% BSA at room
temperature for 1 h with rabbit Ab32 and mouse anti-HA antibodies,
washed intensively and then incubated with the secondary donkey anti-
mouse and anti-rabbit IgG labeled with Texas red and fluorescein
isothiocyanate (FITC), respectively (Jackson Tec.). Both primary and
secondary antibody solutions were pre-cleared by centrifugation at
100 000 g for 10 min. After staining, the coverslips were mounted in
Fluoromount-G (Fisher) containing 1 mg/ml p-phenylenediamine. To
obtain high-resolution three-dimensional images of cells, we used an
imaging workstation consisting of the thermoelectrically cooled charged-
coupled device (CCD) Micromax camera with Sony Interline area array
(Princeton Instruments) and a Nikon Diaphot microscope equipped with
z-step motor and dual filter wheel controlled by QED Imaging or
Intelligent Imaging Innovation (Denver, CO) software. Typically, 15–40
serial two-dimensional images were recorded at 100–200 nm intervals.
A Z-stack of images obtained was deconvoluted using a modification of
the constrained iteration method. Final analysis of all images was
performed using AdobePhotoshop 4.03.
Internalization of [125I]EGF and [125I]transferrin
To monitor [125I]EGF or [125I]transferrin internalization, HeLa cells were
grown in 12-well dishes in the presence or absence of tetracycline. The
cells were incubated in binding medium for 1 h prior to experiments.
To measure the internalization rates, cells were incubated with [125I]EGF
(1 ng/ml) or [125I]transferrin (1 µg/ml) in binding medium at 37°C for
1–6 min. A low concentration of [125I]EGF was used to avoid saturation
of the internalization machinery. After the indicated times, the medium
was aspirated, and the monolayers were washed rapidly three times with
DMEM to remove unbound ligand. The cells were then incubated for
5 min with 0.2 M acetic acid (pH 2.8) containing 0.5 M NaCl at 4°C.
The acid wash was combined with another short rinse in the same buffer,
and used to determine the amount of surface-bound [125I]ligand. The
cells were lysed in 1 M NaOH to determine the intracellular (internalized)](https://image.slidesharecdn.com/f3e1319c-ecbc-49dc-b4f2-a5ade7337b36-160118185808/85/EMBO-9-320.jpg)

