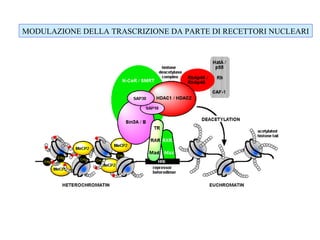
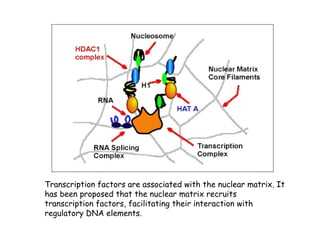
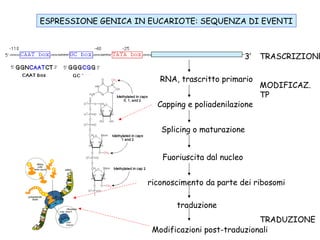
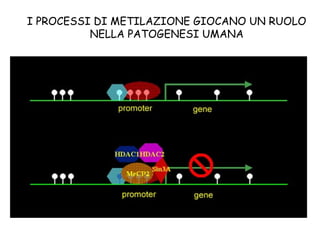
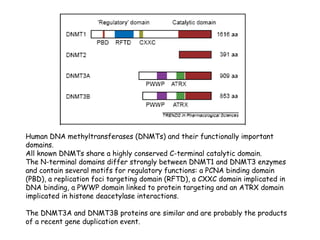
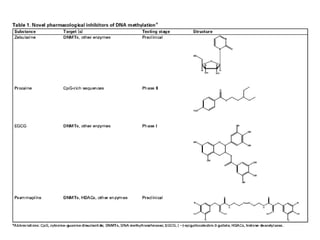
The document discusses methylation processes and their role in human pathogenesis. It first describes how the MeCP2 protein functions in gene silencing through methyl-DNA binding and transcription repression, but that its role is more complex as a regulator rather than strict silencer of transcription. It also discusses how MeCP2 mutations cause Rett syndrome and interactions with other proteins. The role of DNA methylation in cancer is then covered, including global hypomethylation in cancer and hypermethylation of tumor suppressor genes. Finally, the potential of epigenetic therapies targeting DNA methyltransferases and histone deacetylases for cancer treatment is presented.







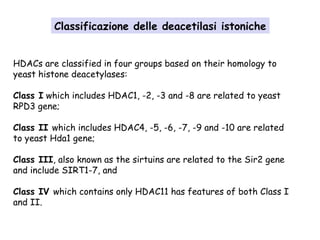
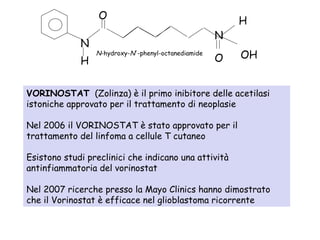
![The “classical” HDIs act exclusively on Class I and Class II HDACs by
binding to the zinc containing catalytic domain of the HDACs. These
classical HDIs fall into several groupings, in order of decreasing
potency:
(i) hyroxamic acids, such as Tricostatine A,
(ii) cyclic tetrapeptides (such as trapoxin B), and the depsipeptides,
(iii) benzamides,
(iv) electrophilic ketones, and
(v) the alophatic acid compounds such as phenylbutyrate and valproic
acid.
"Second generation" HDIs include SAHA/Vorinostat,
Belinostat/PXD101, MS275, LAQ824/LBH589, CI994, and
MGCD0103.
The sirtuin Class III HDACs are NAD+ dependent and are therefore
inhibited by nicotinamides, as well derivatives of NAD,
dihydrocoumarin, naphthopyranone, and 2-hydroxynaphaldehydes.[4]
Classificazione degli inibitori delle istone deacetilasi](https://image.slidesharecdn.com/sindromedirett-130801013803-phpapp01/85/Sindrome-di-rett-16-320.jpg)