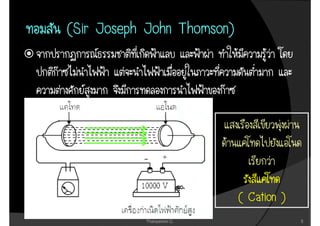
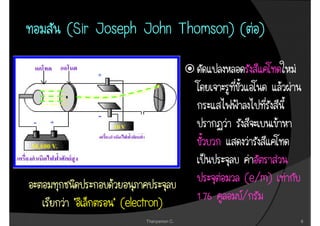
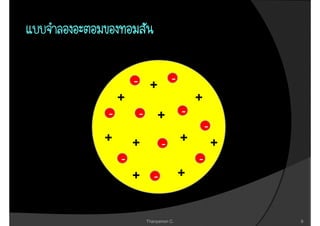
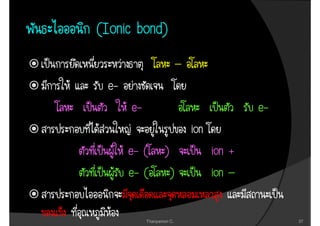
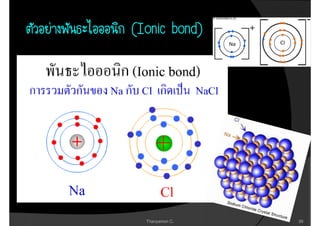
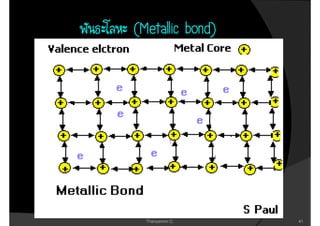
1. The document discusses the early history of atomic theory from Democritus' idea of indivisible atoms to Thomson's discovery of the electron and its mass to charge ratio.
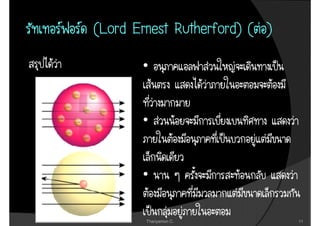
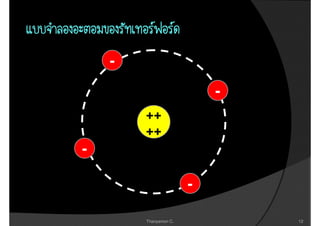
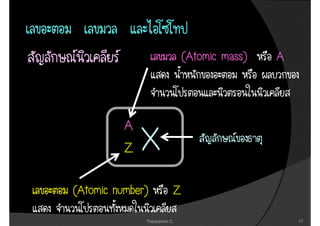
2. It then covers the discoveries of the proton by Goldstein and Millikan, and Rutherford's nuclear model of the atom.
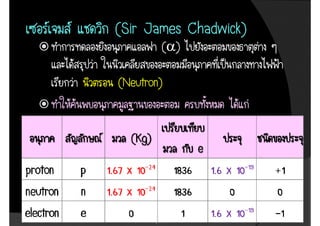
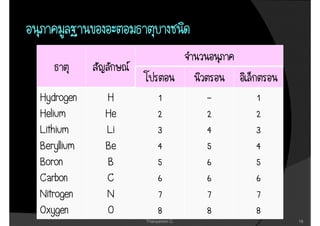
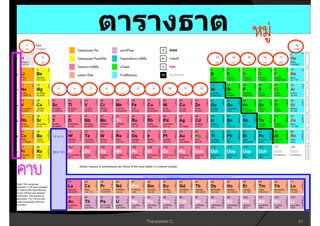
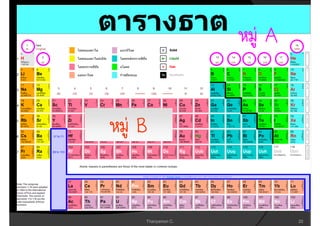
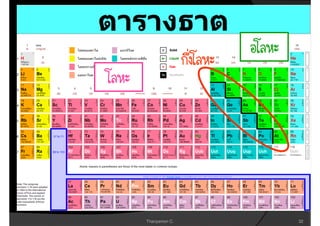
3. The document concludes with brief summaries of Bohr's model of electron shells, Chadwick's discovery of the neutron, and Mendeleev's development of the periodic table.