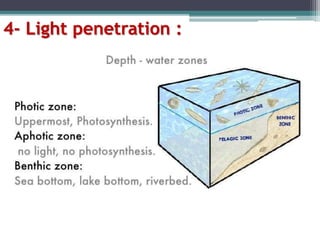
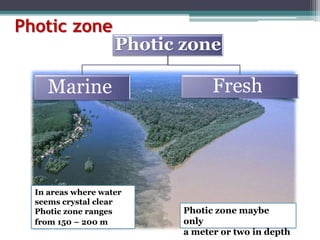
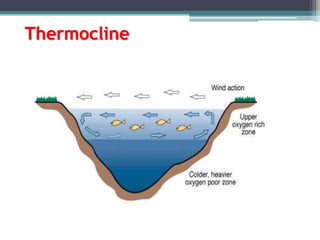
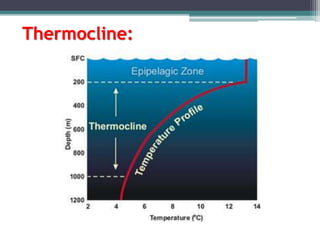
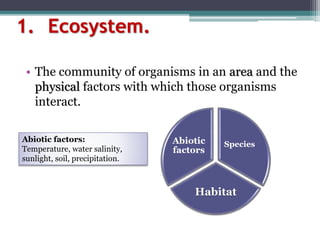
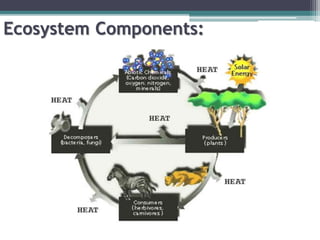
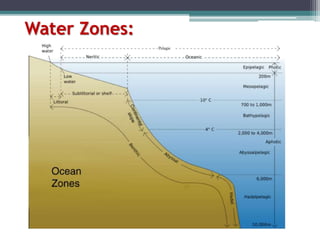
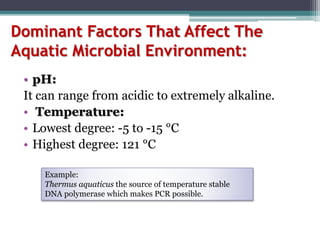
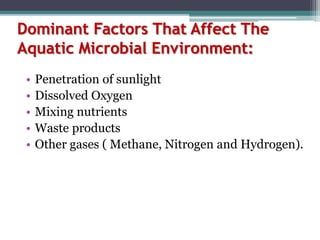
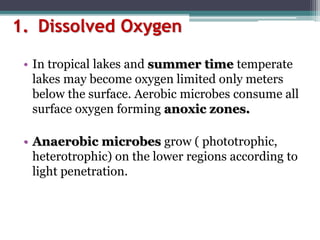
This document provides an overview of microbial marine ecology. It discusses the key components of aquatic ecosystems and how microbes play an important role as decomposers and in maintaining balance. Approximately 75% of the Earth's surface is covered by water, with oceans making up the largest portion. The document outlines the different categories of water environments and zones that microbes can inhabit. It also examines the dominant factors that can affect aquatic microbial communities, such as pH, temperature, light penetration, dissolved oxygen, and carbon dioxide levels. In general, this summary gives a high-level introduction to aquatic microbial habitats and the abiotic factors that influence the diversity of microorganisms found in water.








![Carbonate Equilibrium System
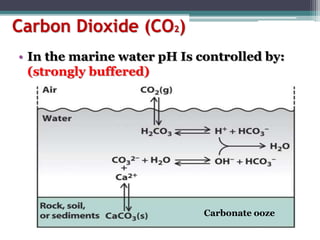
Calcium carbonate, [Ca][CO3] is a very common mineral. Limestone is
one familiar form of calcium carbonate. Acids in acid rain promote
the dissolution of calcium carbonate by reacting with the carbonate
anion.
One that is important in surface waters is the
carbonic acid/bicarbonate buffer.](https://image.slidesharecdn.com/7de52009-b12d-41be-b1b1-dbc8c4ed84b2-160422111809/85/Microbial-Marine-Ecology-16-320.jpg)