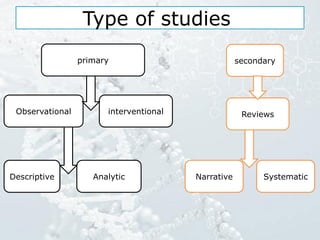
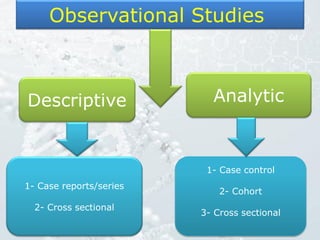
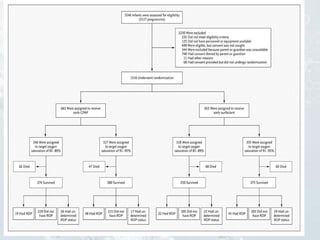
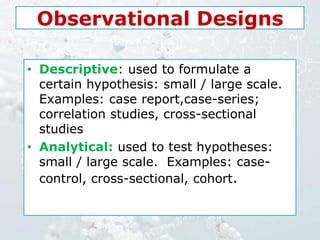
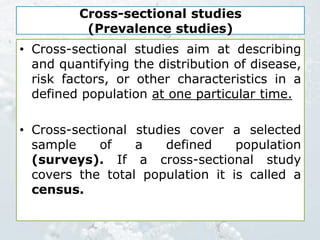
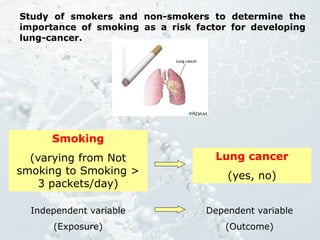
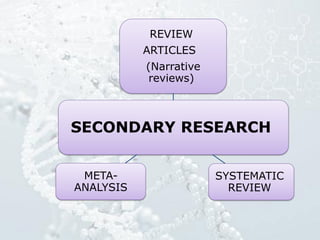
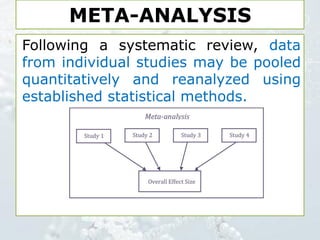
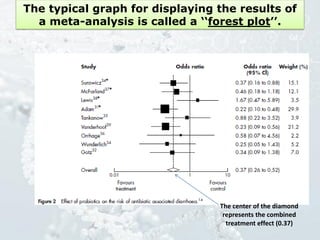
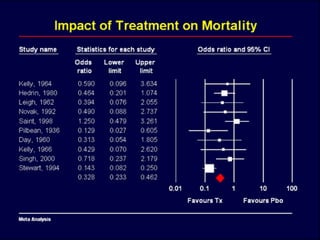
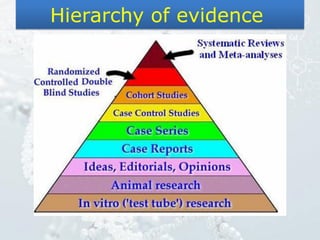
1. The document discusses various types of clinical research studies including observational studies like case reports, cross-sectional studies, and analytical studies like case-control and cohort studies.
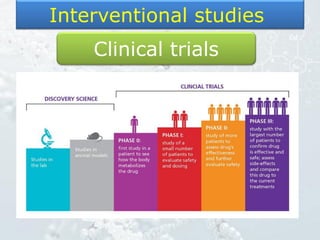
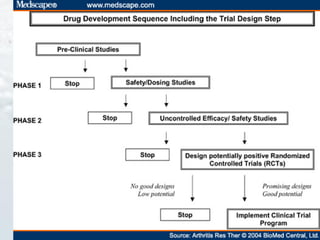
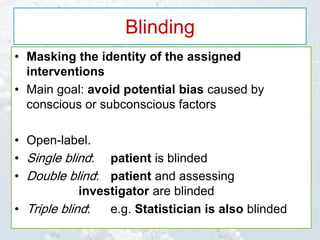
2. Randomized controlled trials are described as the gold standard for clinical trials, with randomization and blinding methods explained to reduce bias.
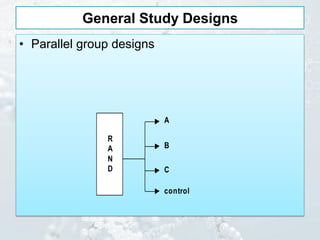
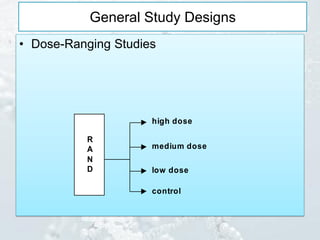
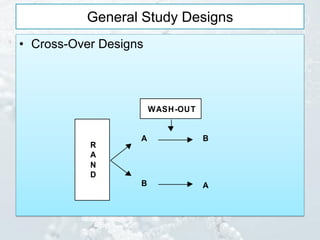
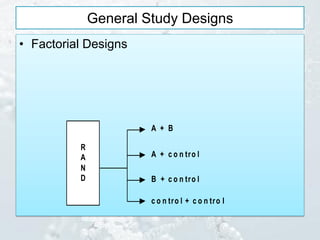
3. Common study designs like parallel group, dose-ranging, cross-over, and factorial designs are summarized along with an example of the Physician's Health Study factorial design.