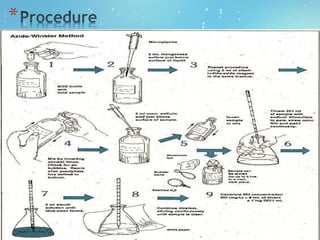
The document explains the factors affecting dissolved oxygen (DO) concentrations in water, including chemical reactions, temperature, pressure, light penetration, and water turbulence. It emphasizes the importance of measuring DO for assessing water quality and provides a detailed procedure for conducting the test using manganous sulfate and sodium thiosulfate. Aquatic plants and animals rely on DO for survival, and insufficient levels can be detrimental to their health.