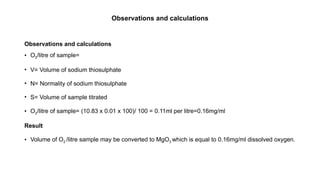
This document describes the Winkler method for determining the amount of dissolved oxygen in a water sample through titration. It outlines the principles, apparatus, reagents, procedures, observations, calculations, and safety precautions involved in the experiment. The results indicated an average dissolved oxygen concentration of 0.16 mg/ml in the sample analyzed.