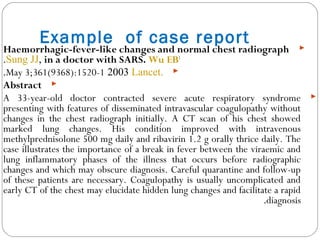
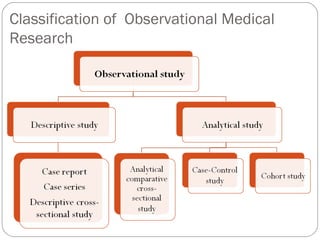
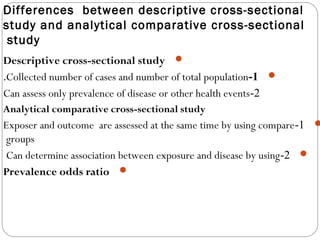
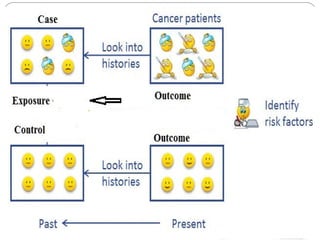
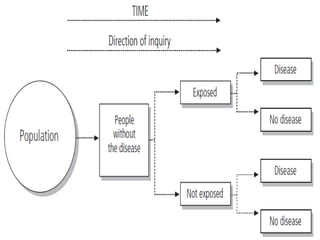
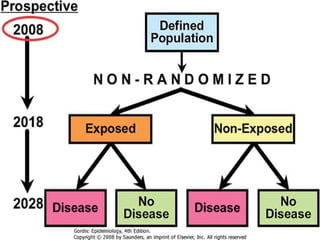
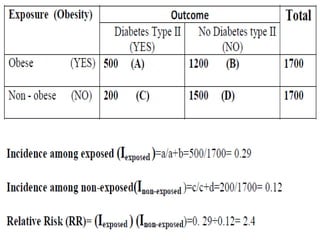
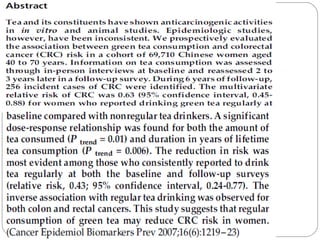
This document defines and classifies different types of medical research. It begins by defining research as a systematic and organized method for finding answers to questions. Medical research is then classified into primary research, which involves collecting new data, and secondary research, which involves collecting existing data from other researchers. Observational research is further divided into descriptive studies, which observe characteristics without intervention, and analytical studies, which attempt to establish causes or risk factors. Specific types of descriptive and analytical studies discussed include case reports, case series, cross-sectional studies, and case-control studies. Key aspects and examples of each type of study are provided.