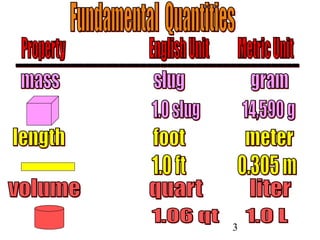
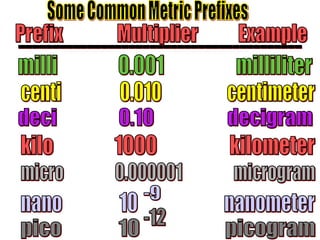
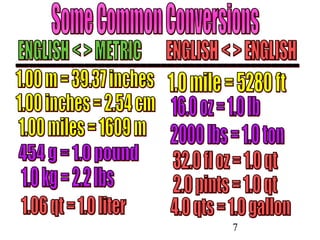
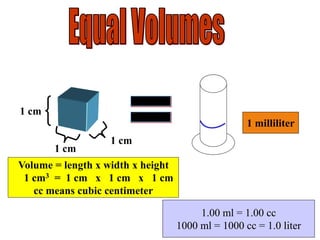
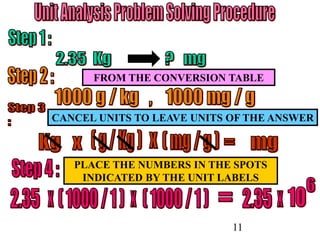
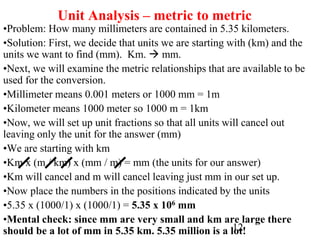
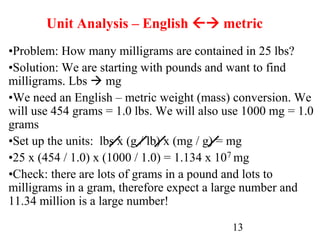
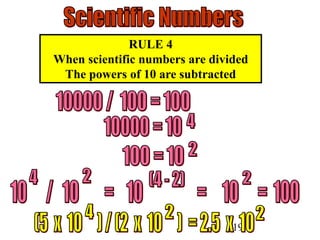
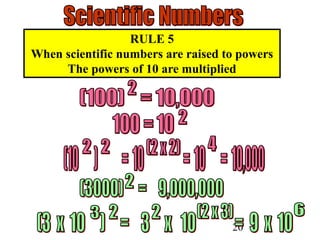
The document outlines key principles of measurement, emphasizing the significance of using standard units such as metric and English systems. It explains how metric units can be subdivided using prefixes and illustrates unit conversion methods, specifically through dimensional analysis. Additionally, the document introduces scientific notation for handling very large or small numbers, detailing rules for manipulating these numbers in calculations.