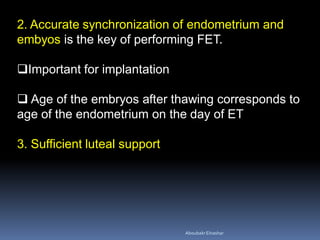
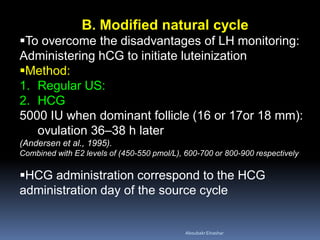
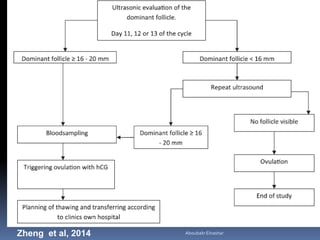
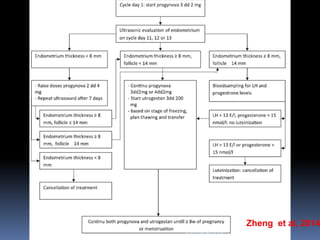
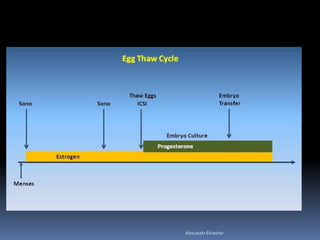
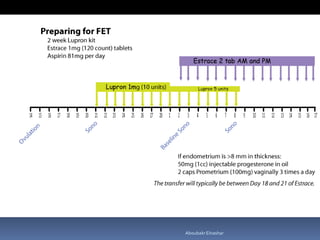
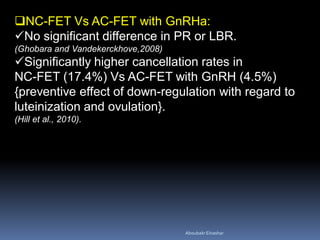
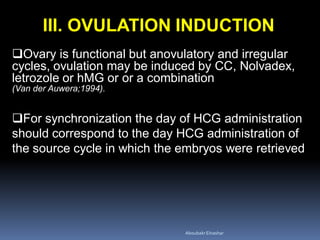
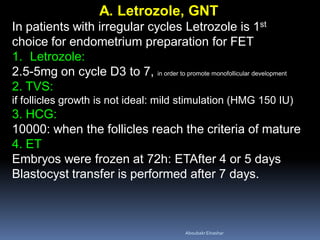
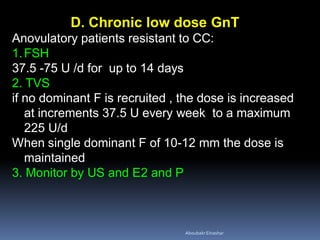
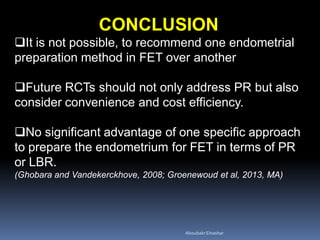
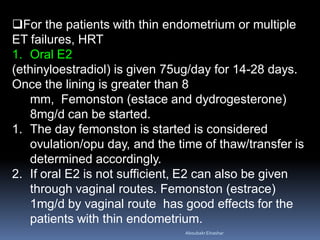
This document discusses different methods for endometrial preparation in frozen embryo transfer (FET) cycles. It describes natural cycle FET, which can be done through a true natural cycle or modified natural cycle with an HCG trigger. It also outlines artificial/hormone replacement cycle FET, where estrogen and progesterone are administered without GnRH agonists in patients with remaining ovarian function. The key points are that the endometrium must be adequately prepared prior to embryo transfer, and the age of the embryos after thawing should correspond to the developmental age of the endometrium. The best method varies between patients and there is no clear consensus.