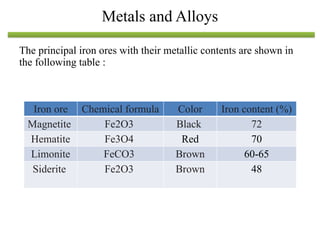
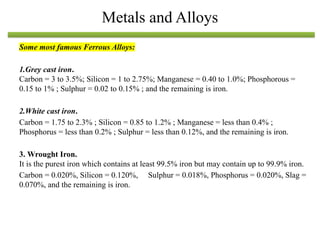
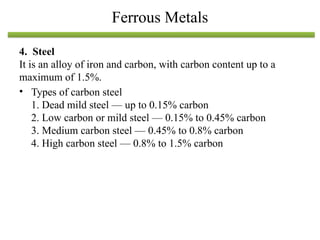
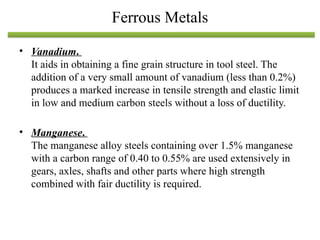
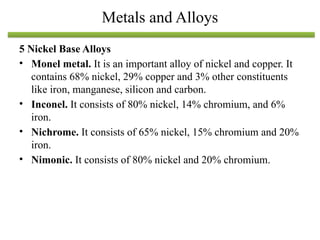
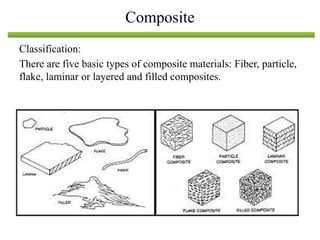
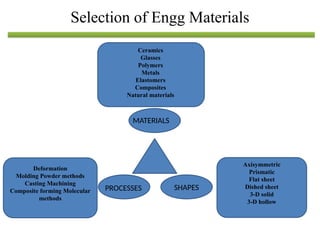
The document discusses engineering materials, categorizing them into metals and alloys, ceramics, polymers, composites, and advanced materials. It outlines the properties, classifications, and applications of these materials, emphasizing the importance of material selection based on factors like cost and mechanical properties. The properties of materials, such as strength, ductility, and toughness, are critical for various engineering applications.