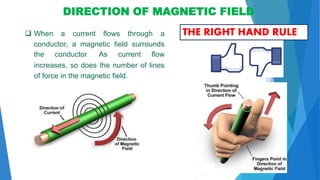
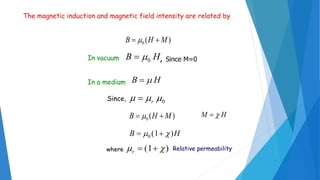
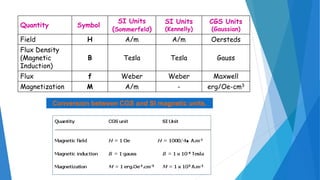
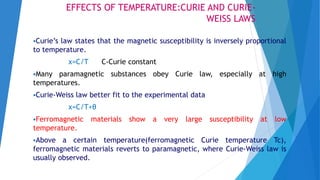
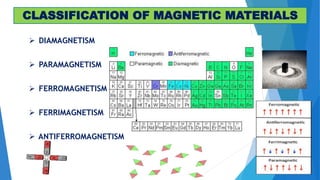
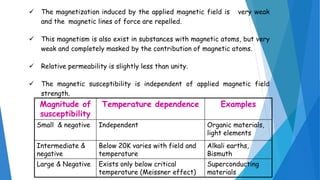
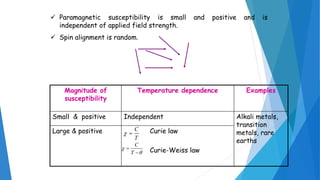
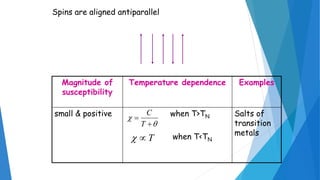
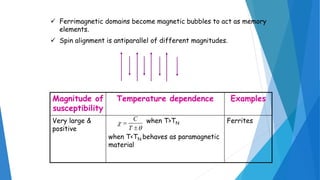
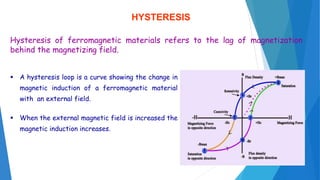
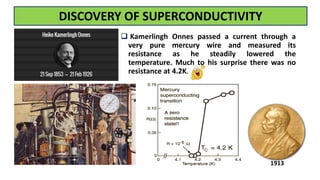
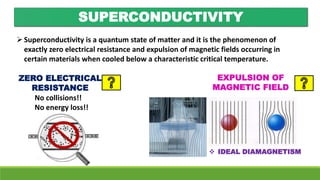
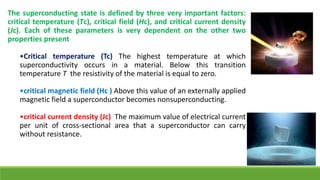
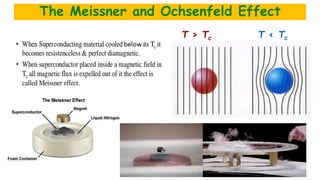
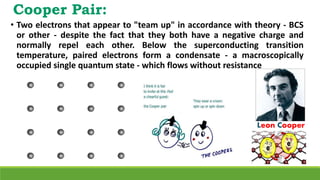
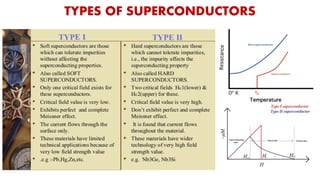
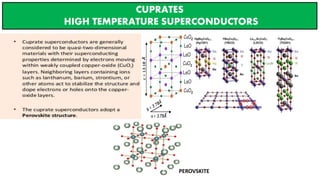
The document discusses the fundamental concepts of magnetism, including the structure of atoms and the behavior of electrons in various magnetic materials such as diamagnetic, paramagnetic, and ferromagnetic substances. It also explains the principles of superconductivity, including critical temperatures, behaviors under magnetic fields, and the BCS theory that describes how electron pairs operate without resistance in superconductors. Furthermore, it details the discovery of high-temperature superconductors and their applications in technologies like maglev trains and telecommunications.