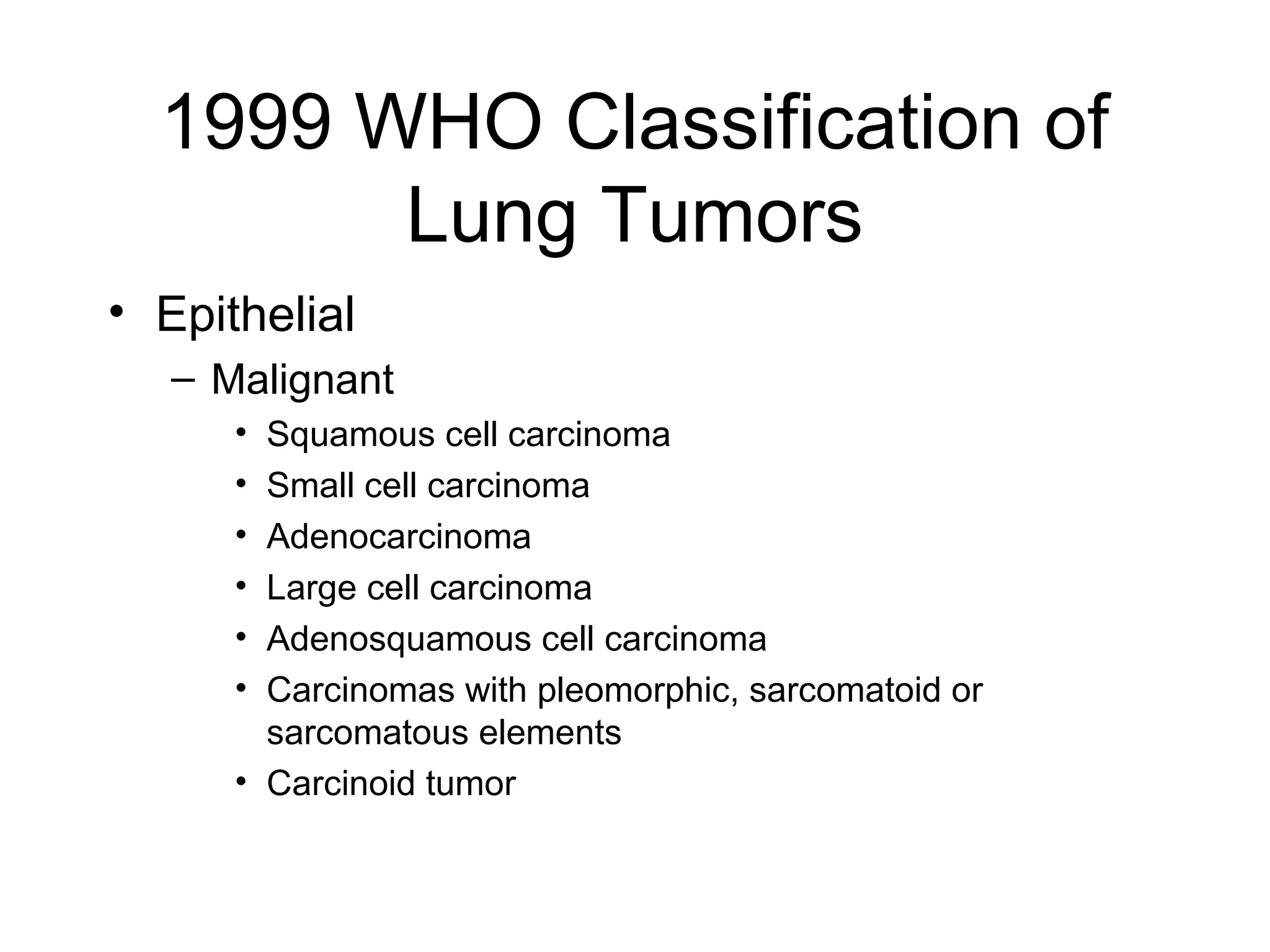
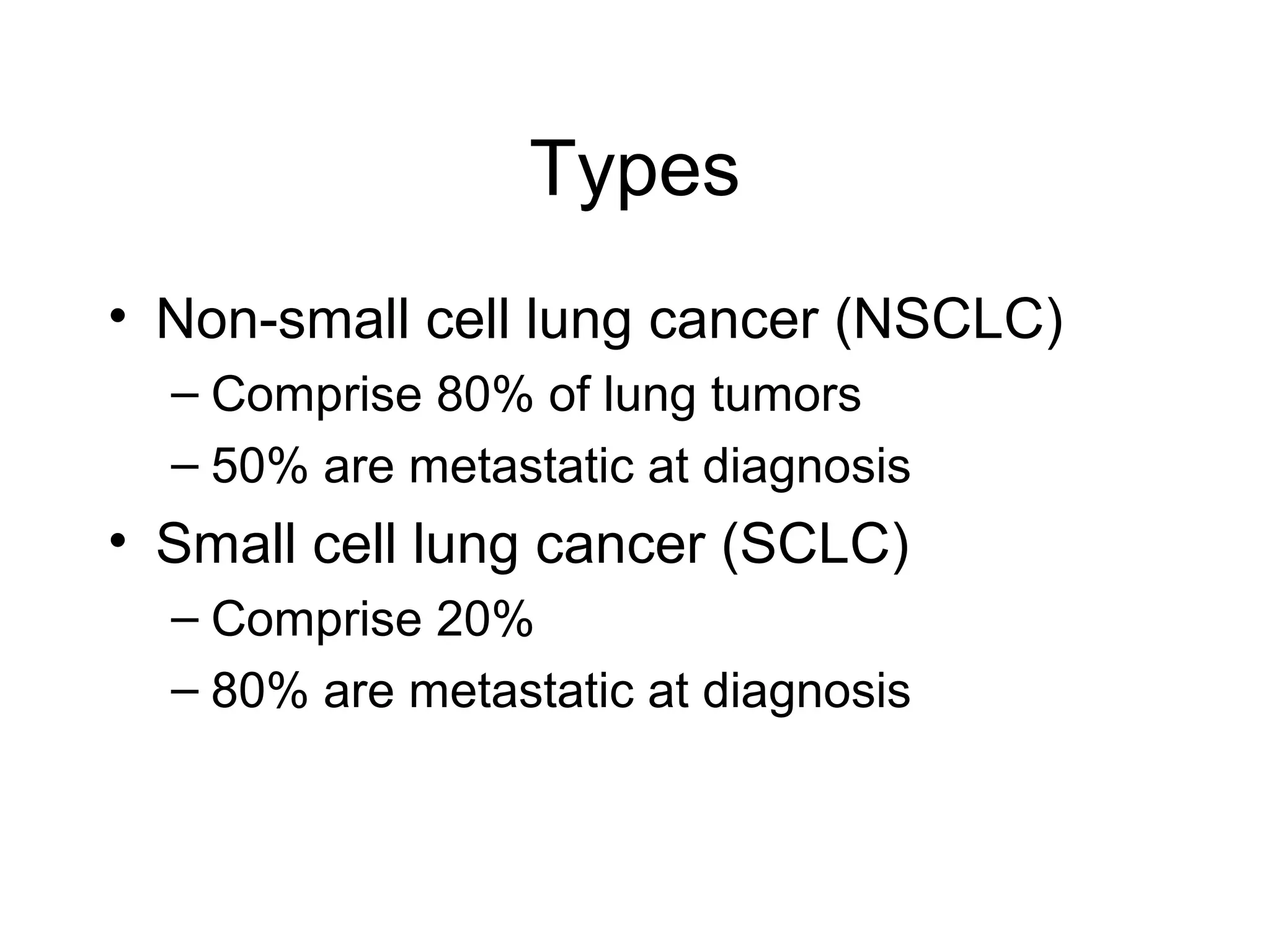
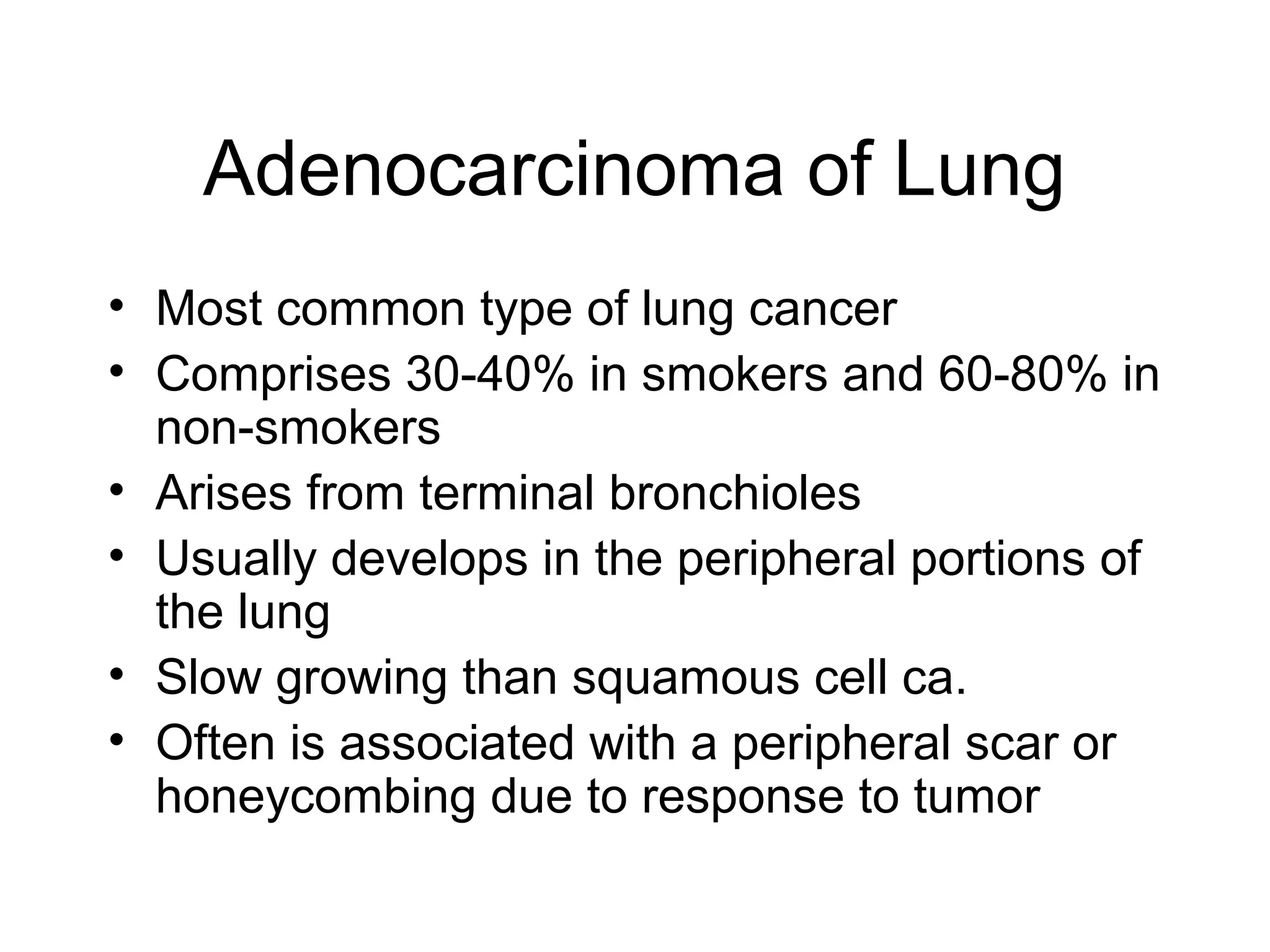
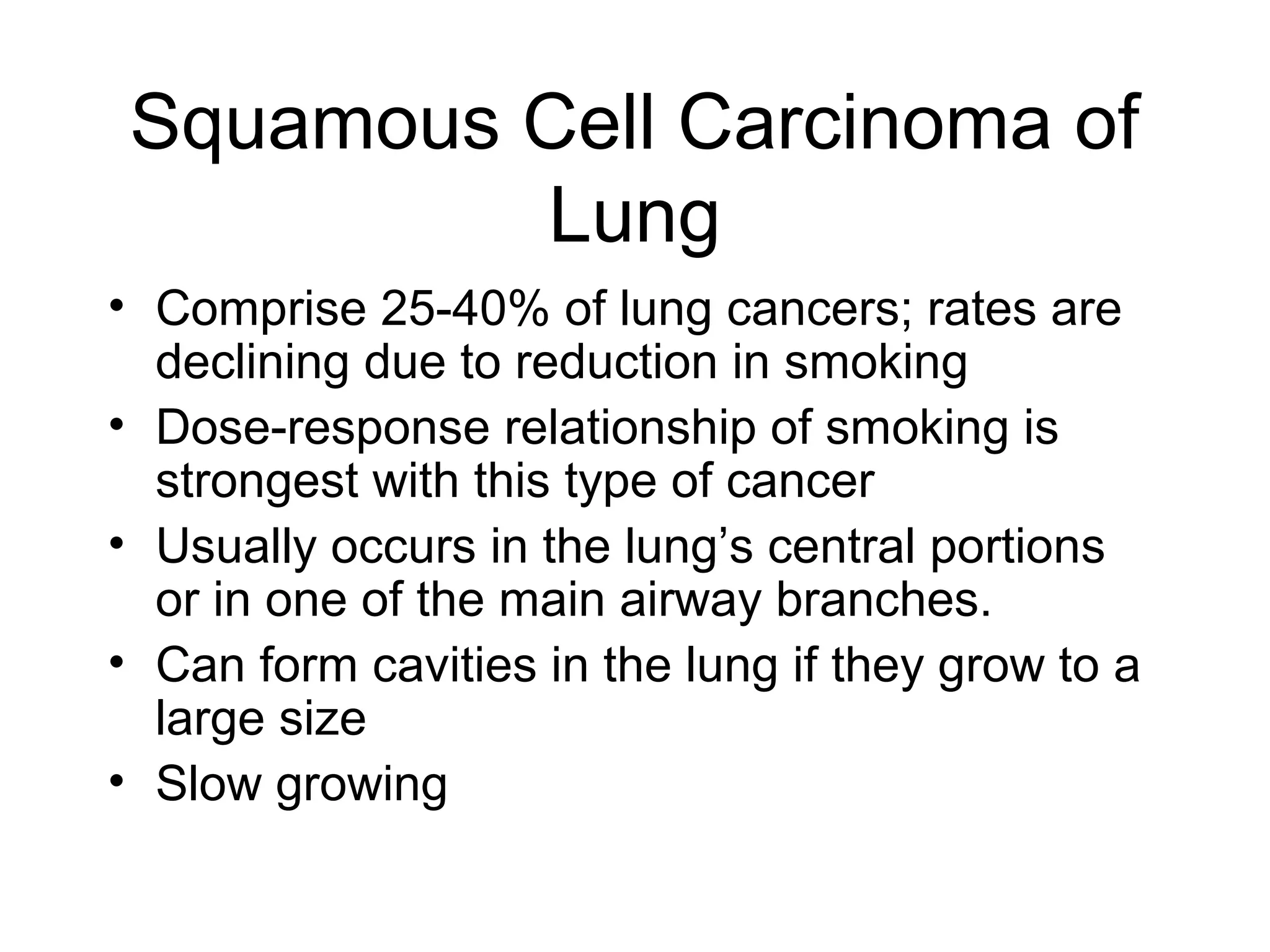
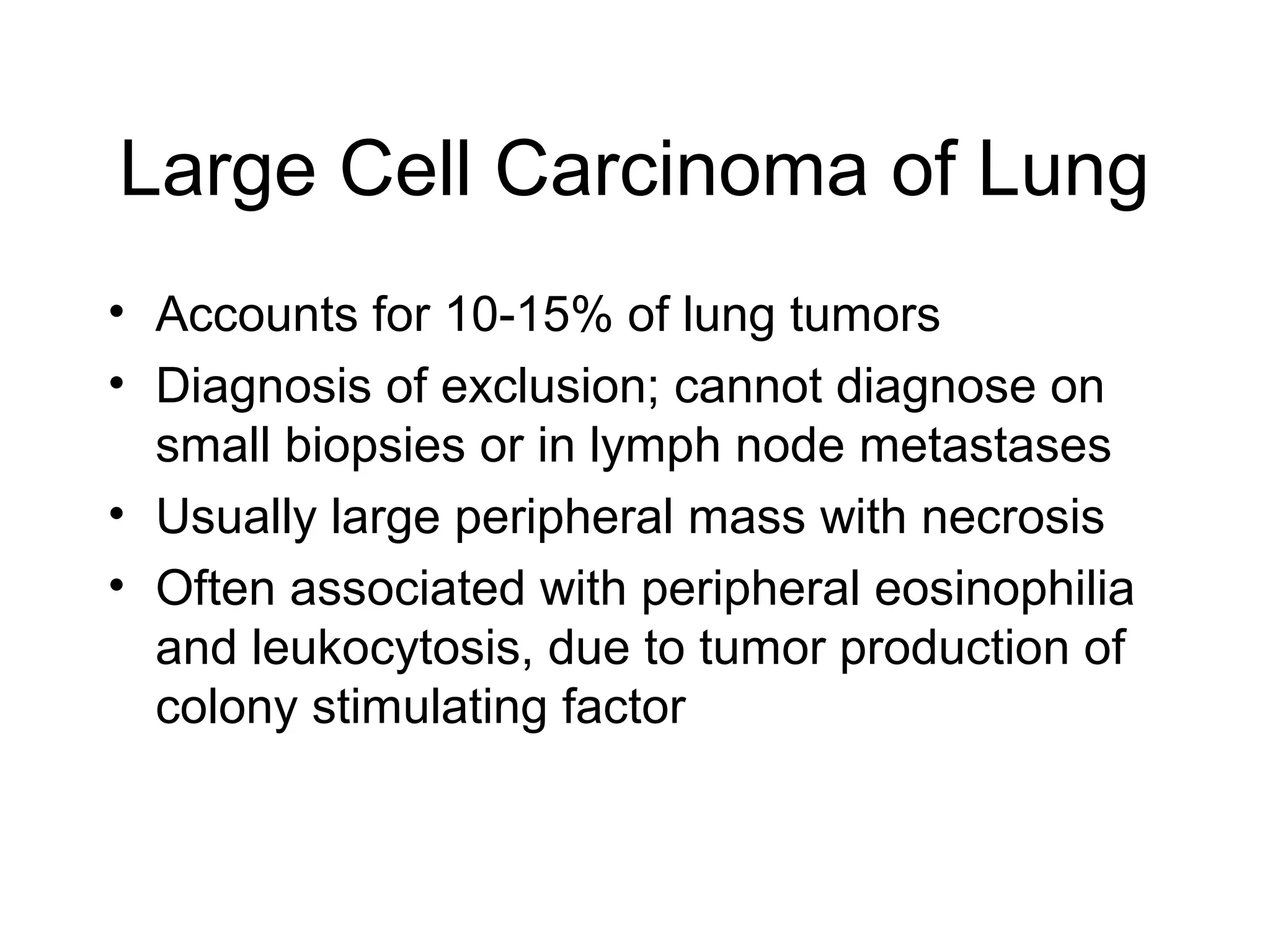
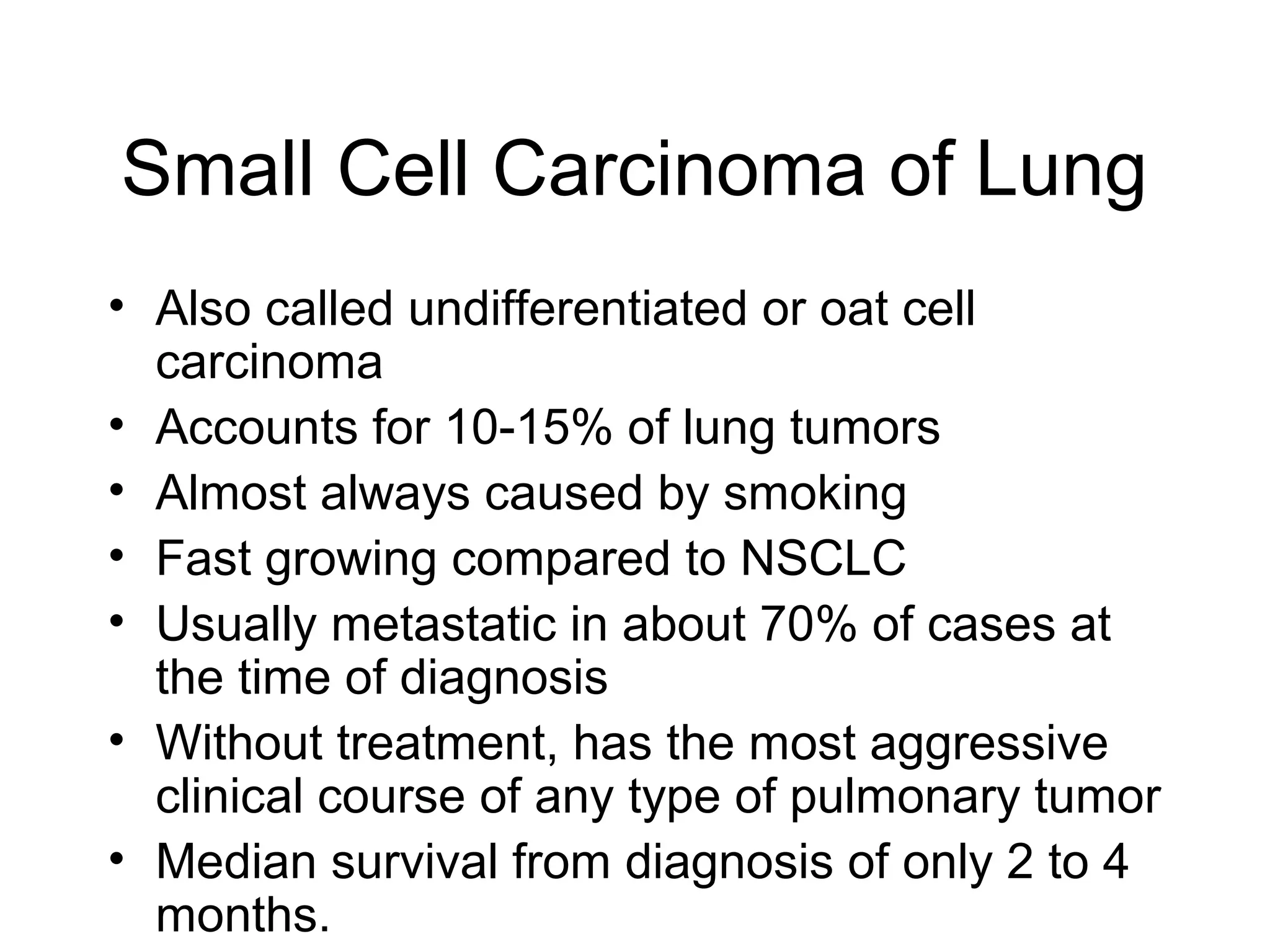
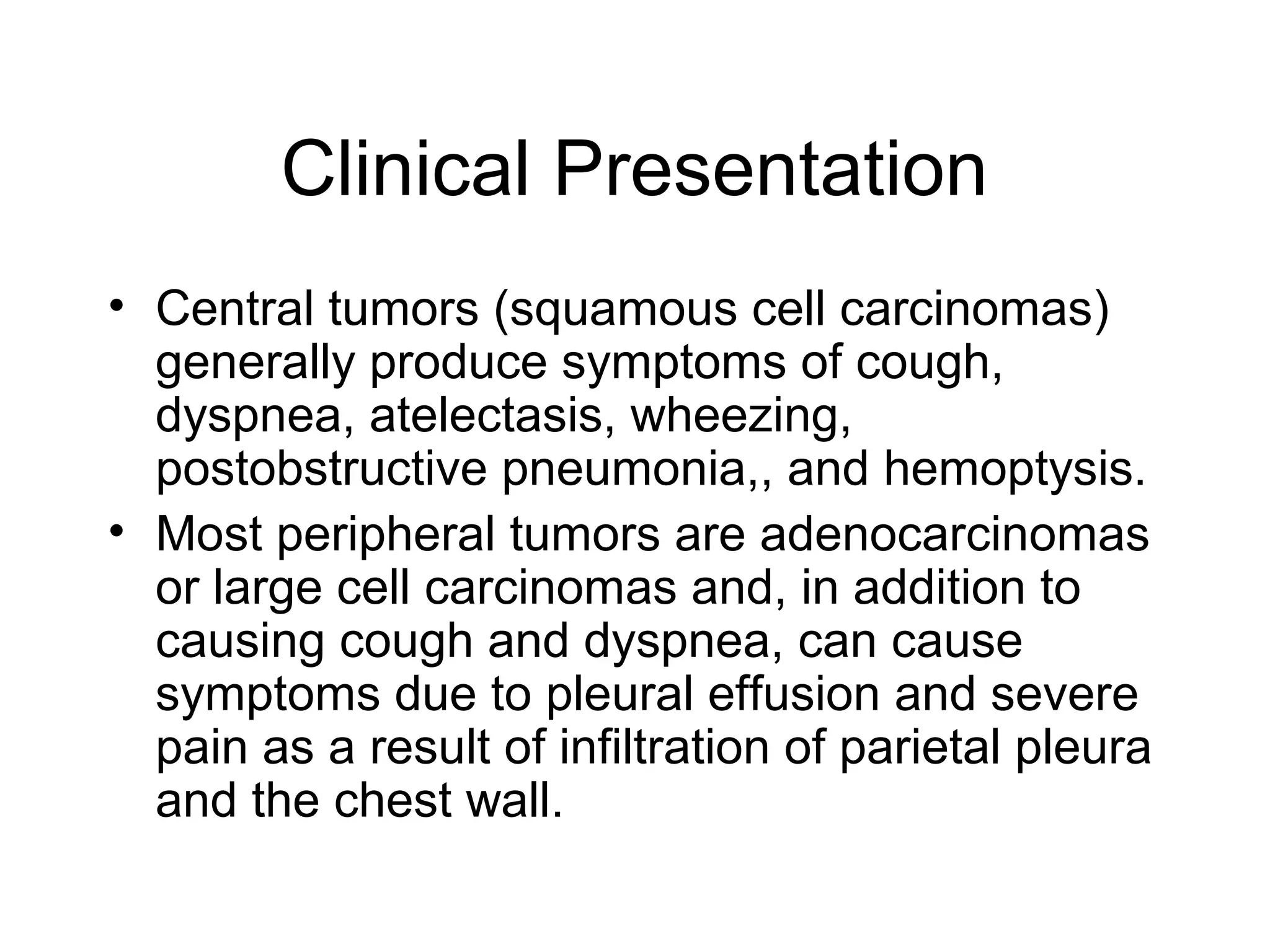
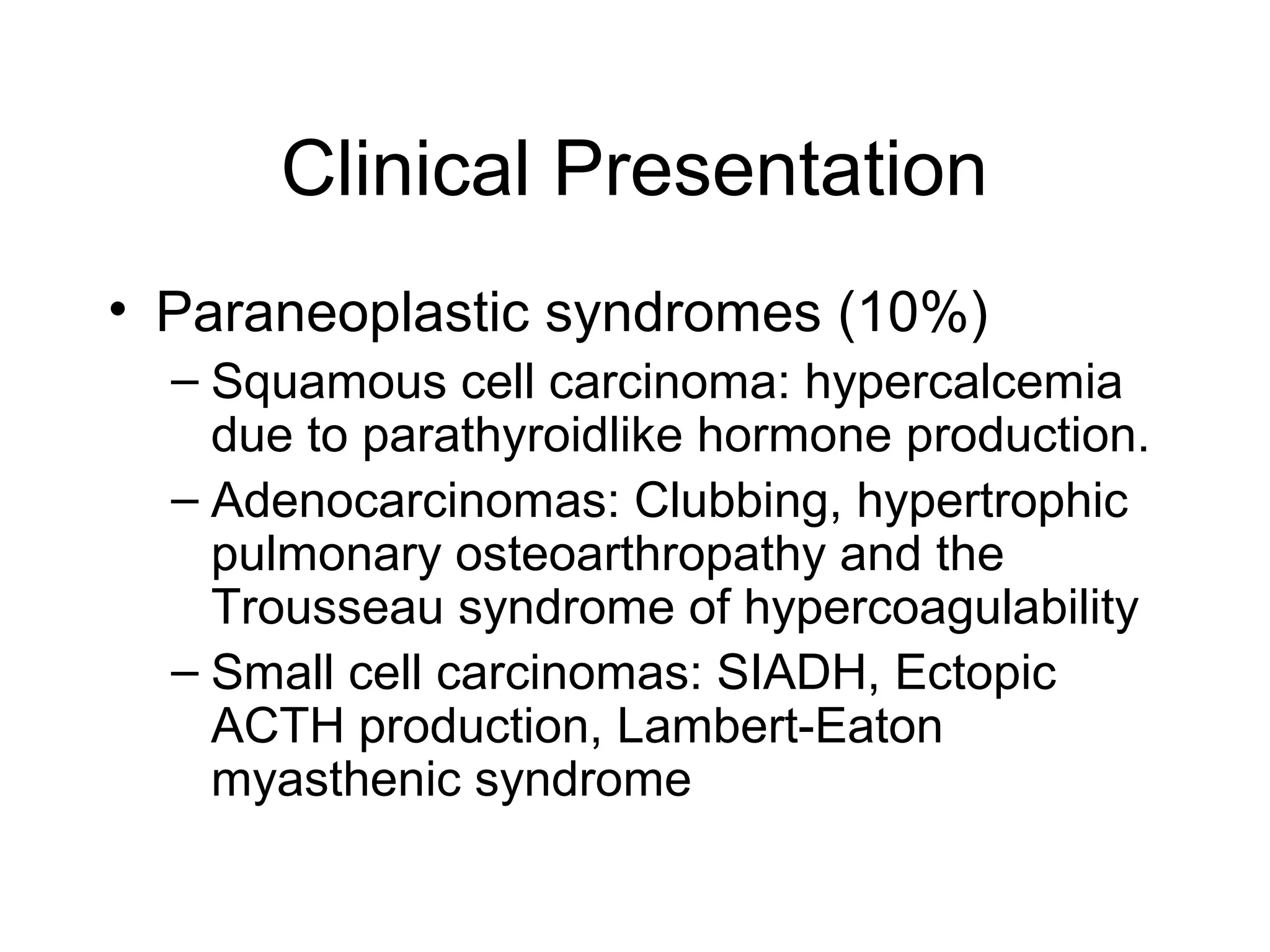
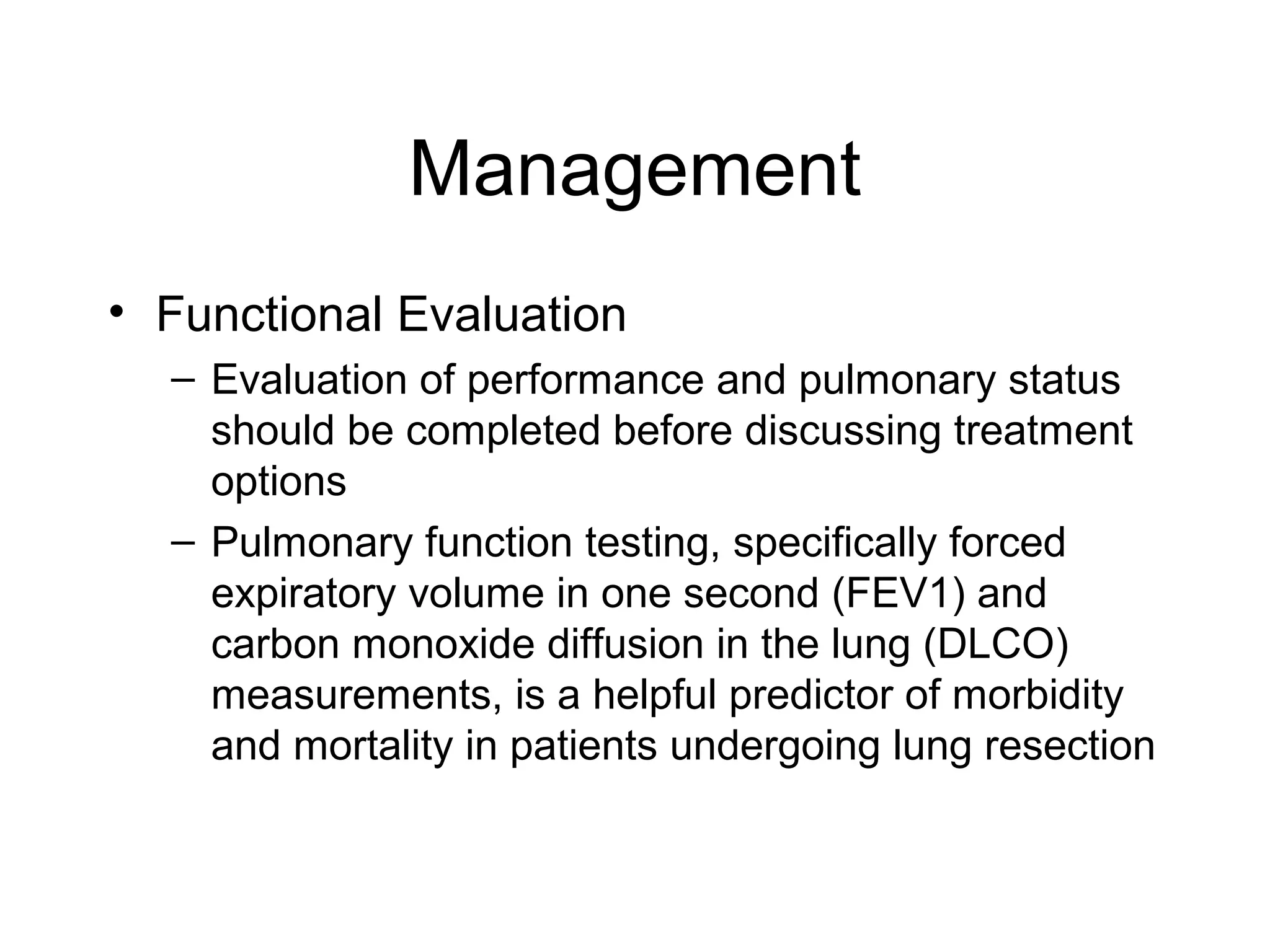
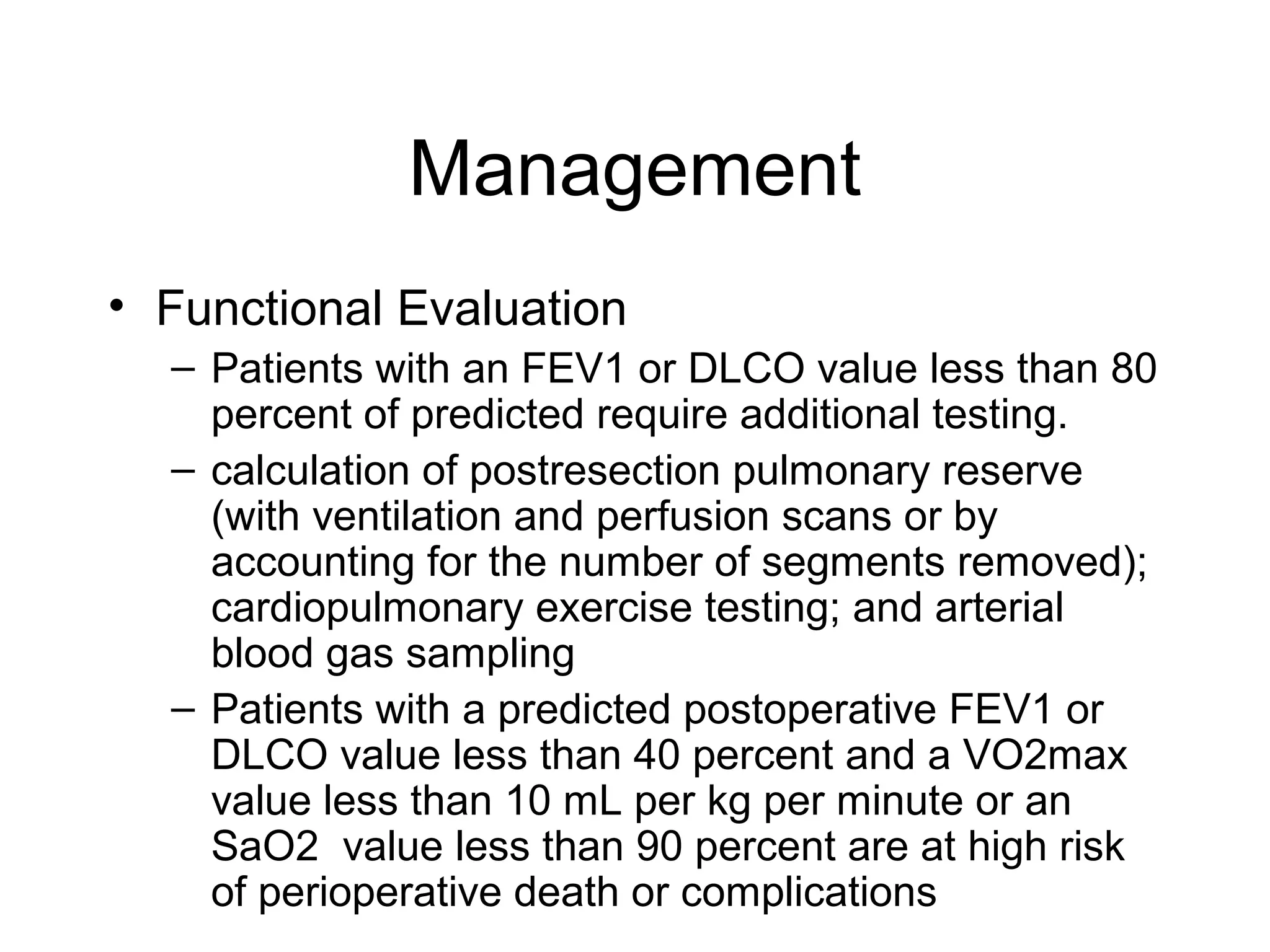
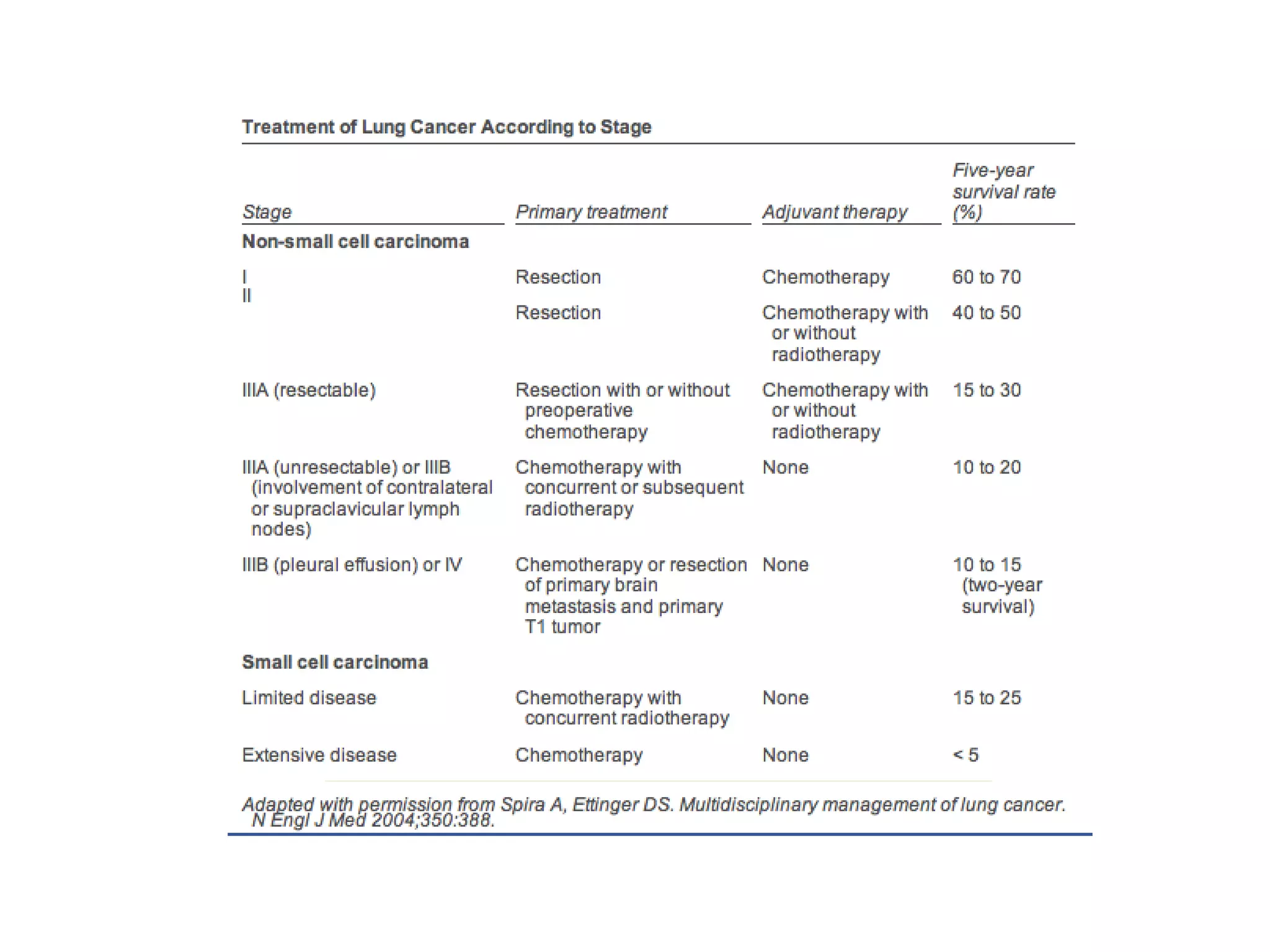
- Lung cancer is a leading cause of cancer death in the US, with an estimated 215,000 new cases and 162,000 deaths in 2008. Non-small cell lung cancer (NSCLC) accounts for 80% of cases, while small cell lung cancer (SCLC) makes up 20%.
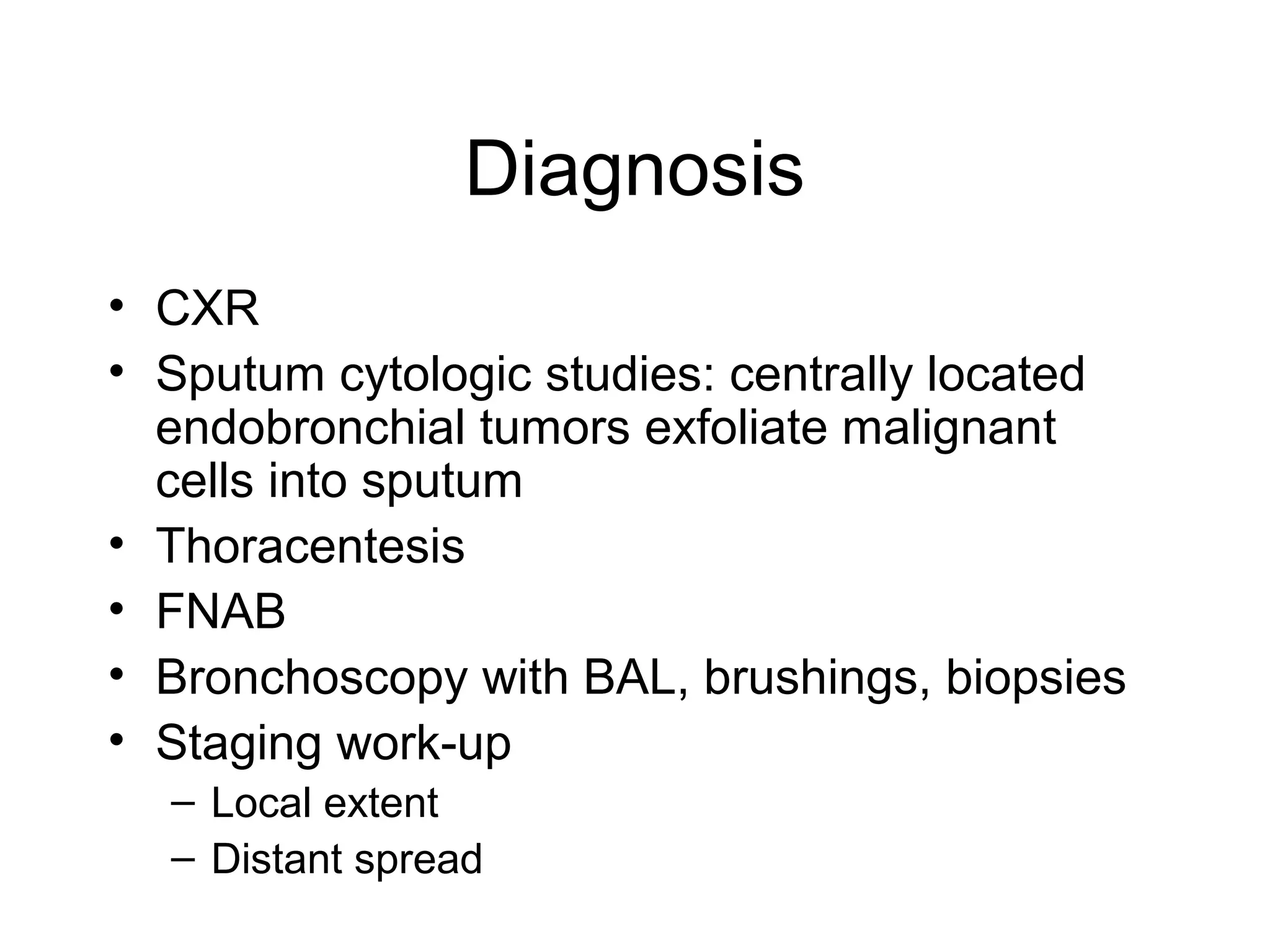
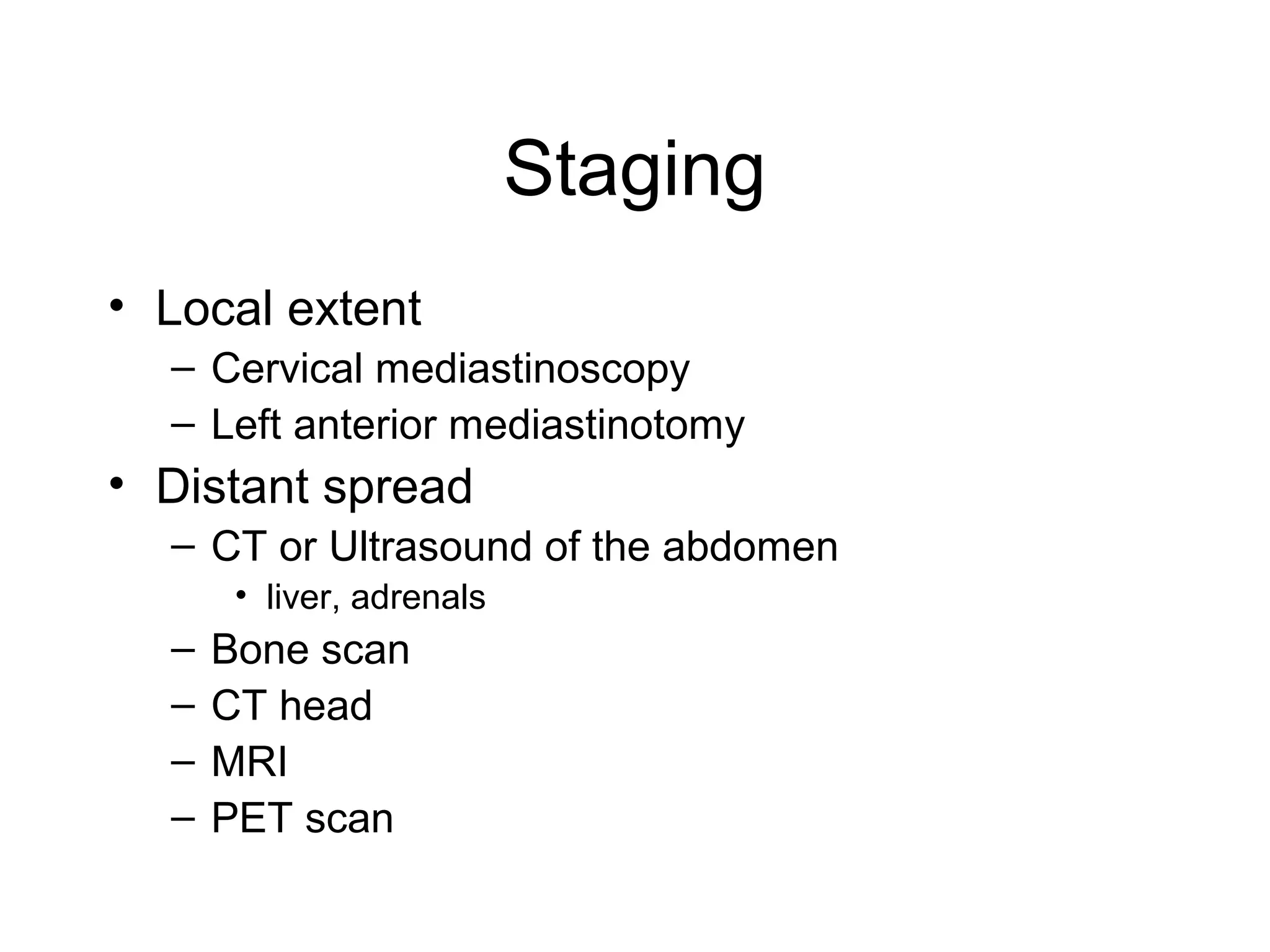
- Smoking is the greatest risk factor, responsible for 87% of lung cancer deaths. Other risk factors include exposure to radon, asbestos, or other gases/particles. Symptoms vary depending on location and stage of cancer but often include cough, dyspnea, chest pain, and weight loss. Diagnosis involves imaging tests, sputum/biopsy analysis, and functional testing to evaluate treatment eligibility