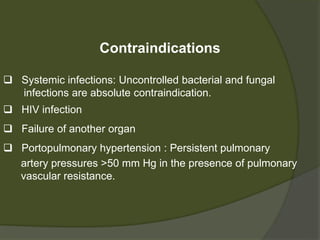
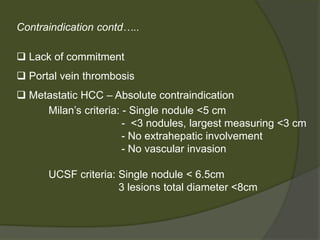
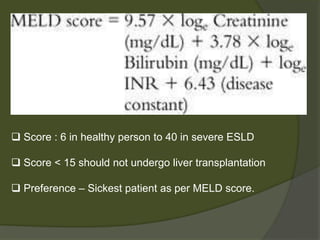
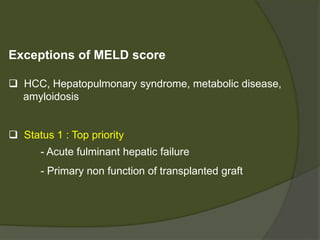
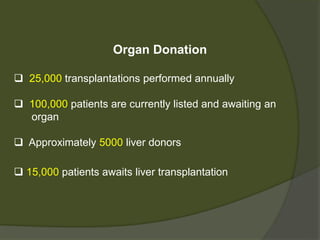
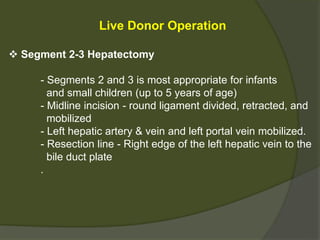
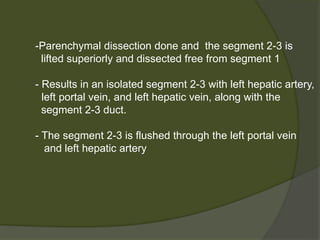
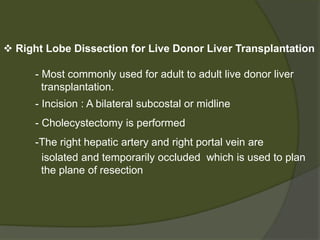
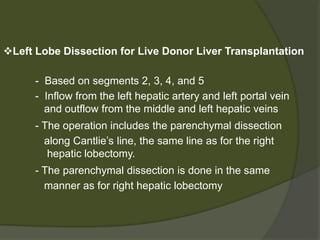
1) Liver transplantation involves replacing a diseased liver with a healthy donor liver. It is used to treat end-stage liver diseases like cirrhosis and liver failure.
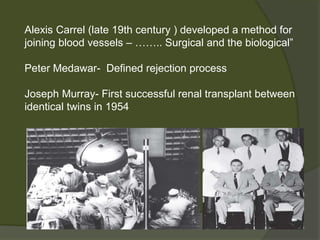
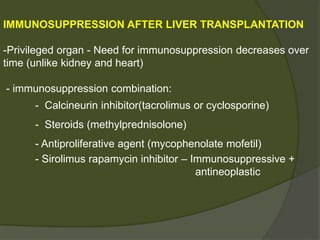
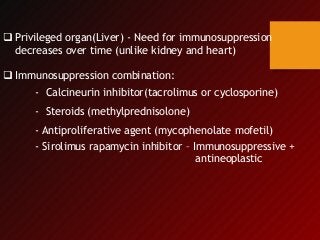
2) The first successful liver transplant was performed in 1963. Advances like immunosuppressive drugs and the MELD scoring system to prioritize recipients have increased transplantation success rates.
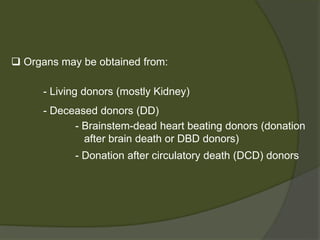
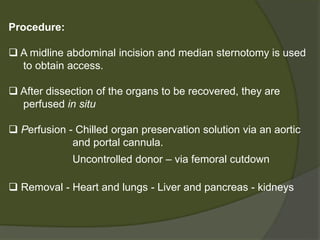
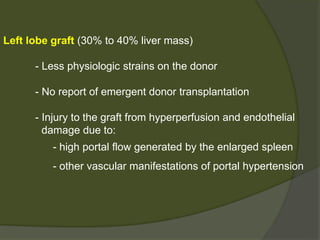
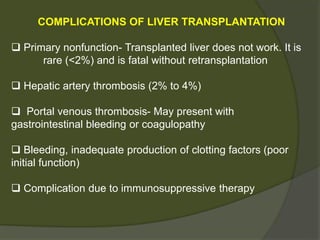
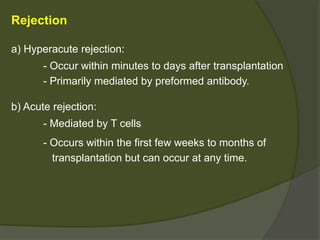
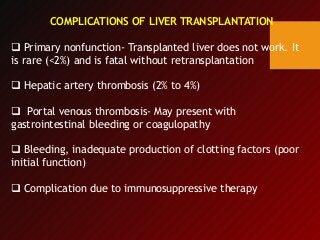
3) Liver transplants can involve whole organs from deceased donors or partial grafts from living donors. Careful donor and recipient evaluation is required to minimize risks of rejection or complications.