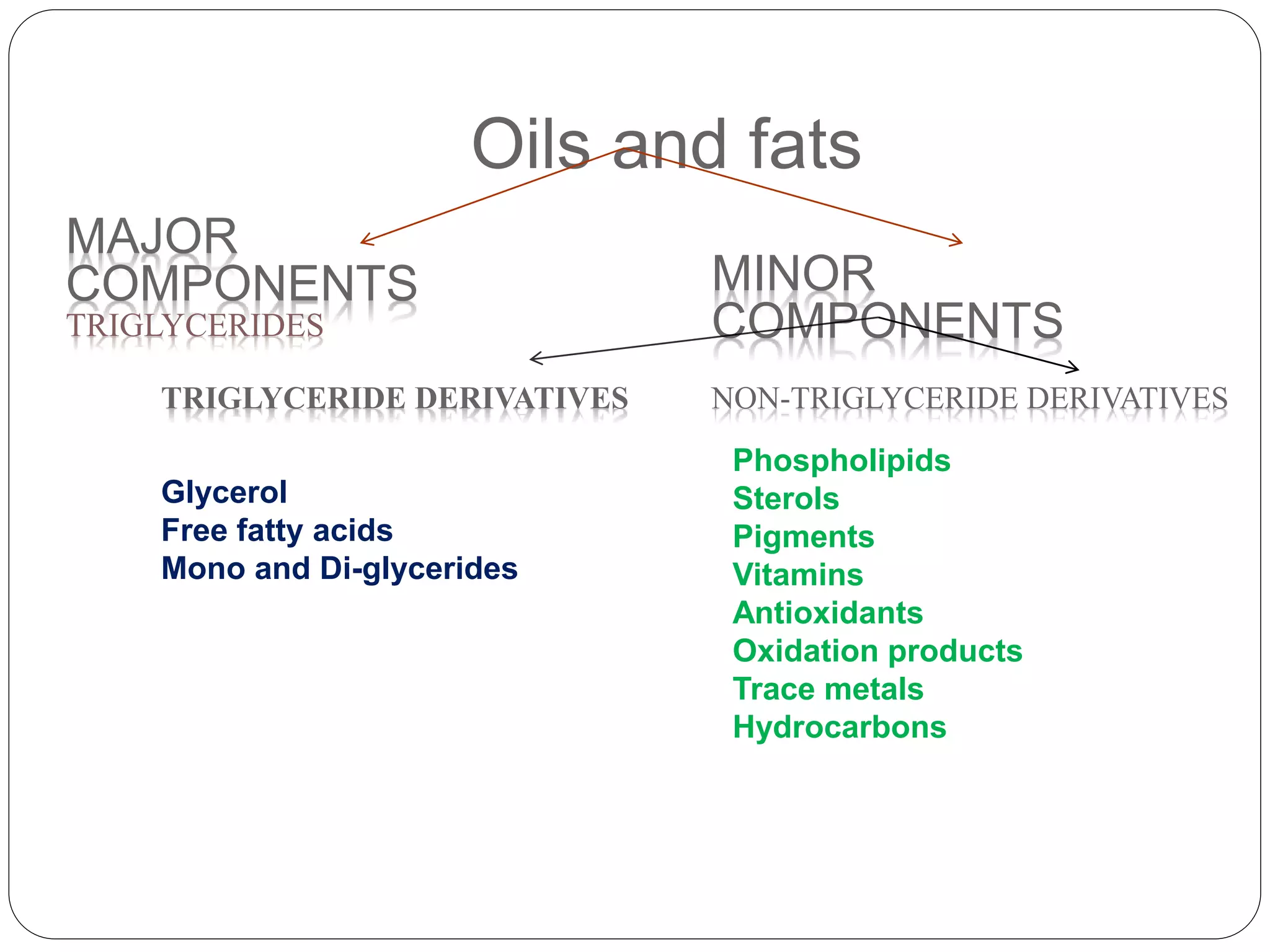
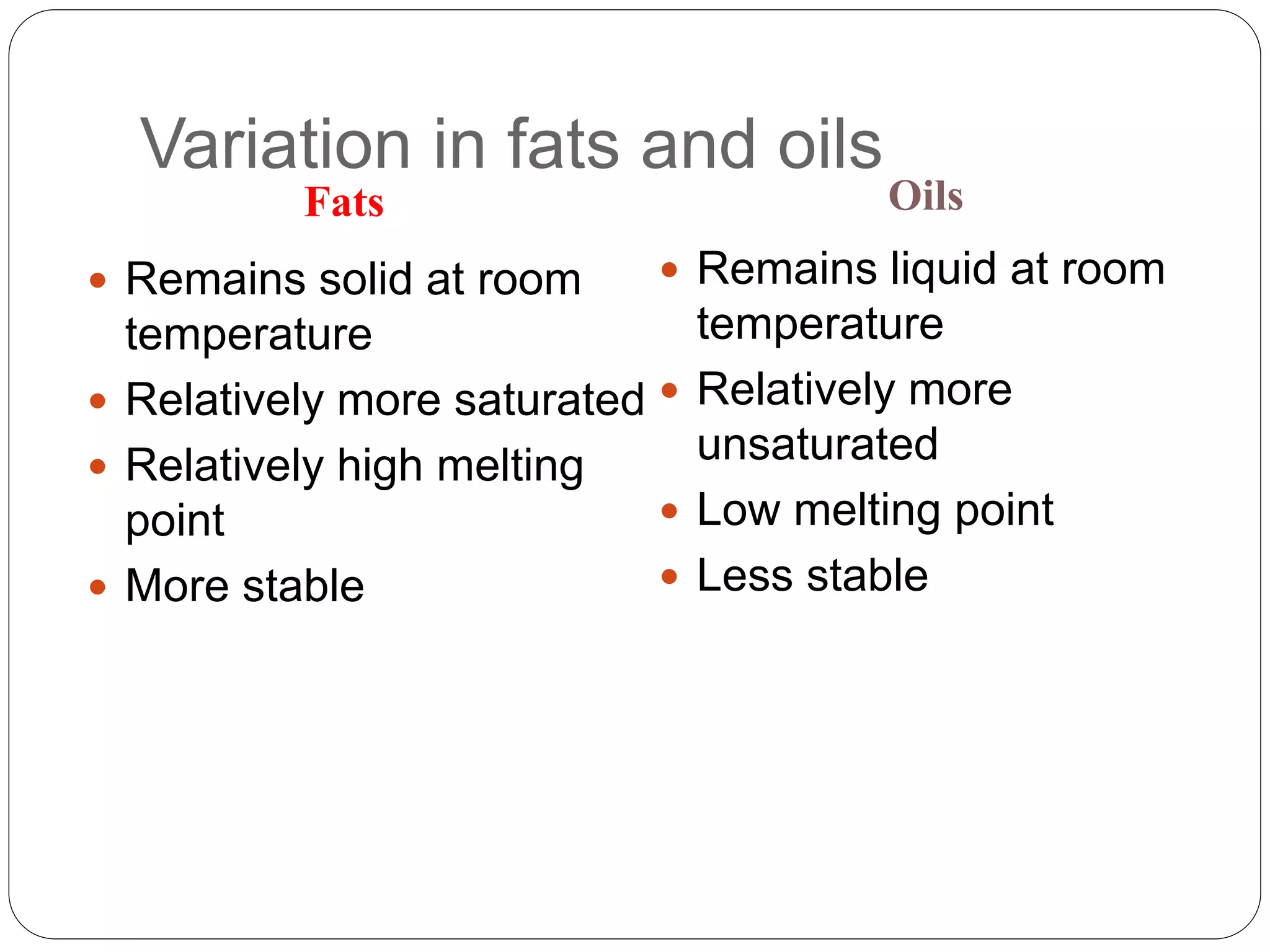
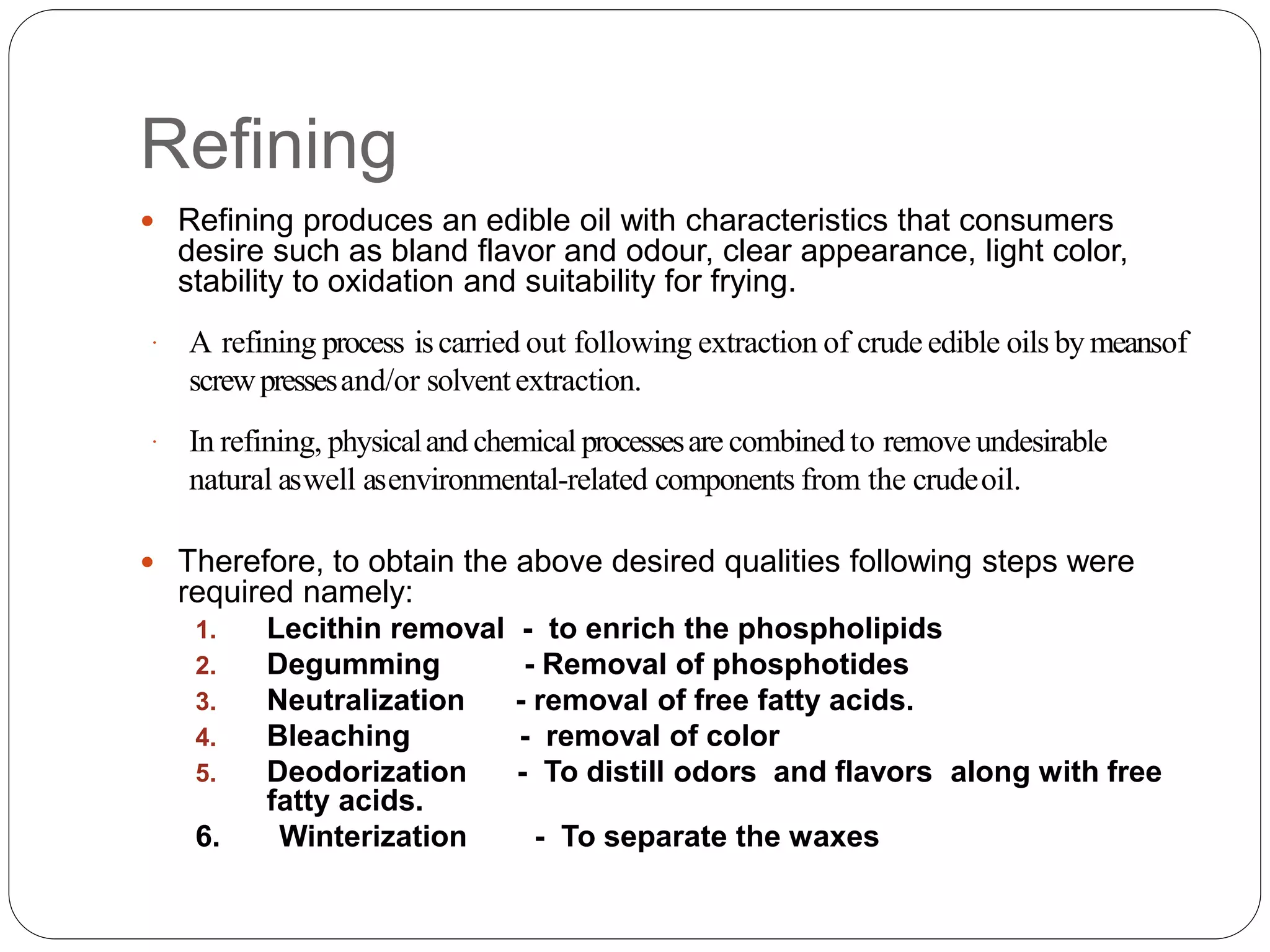
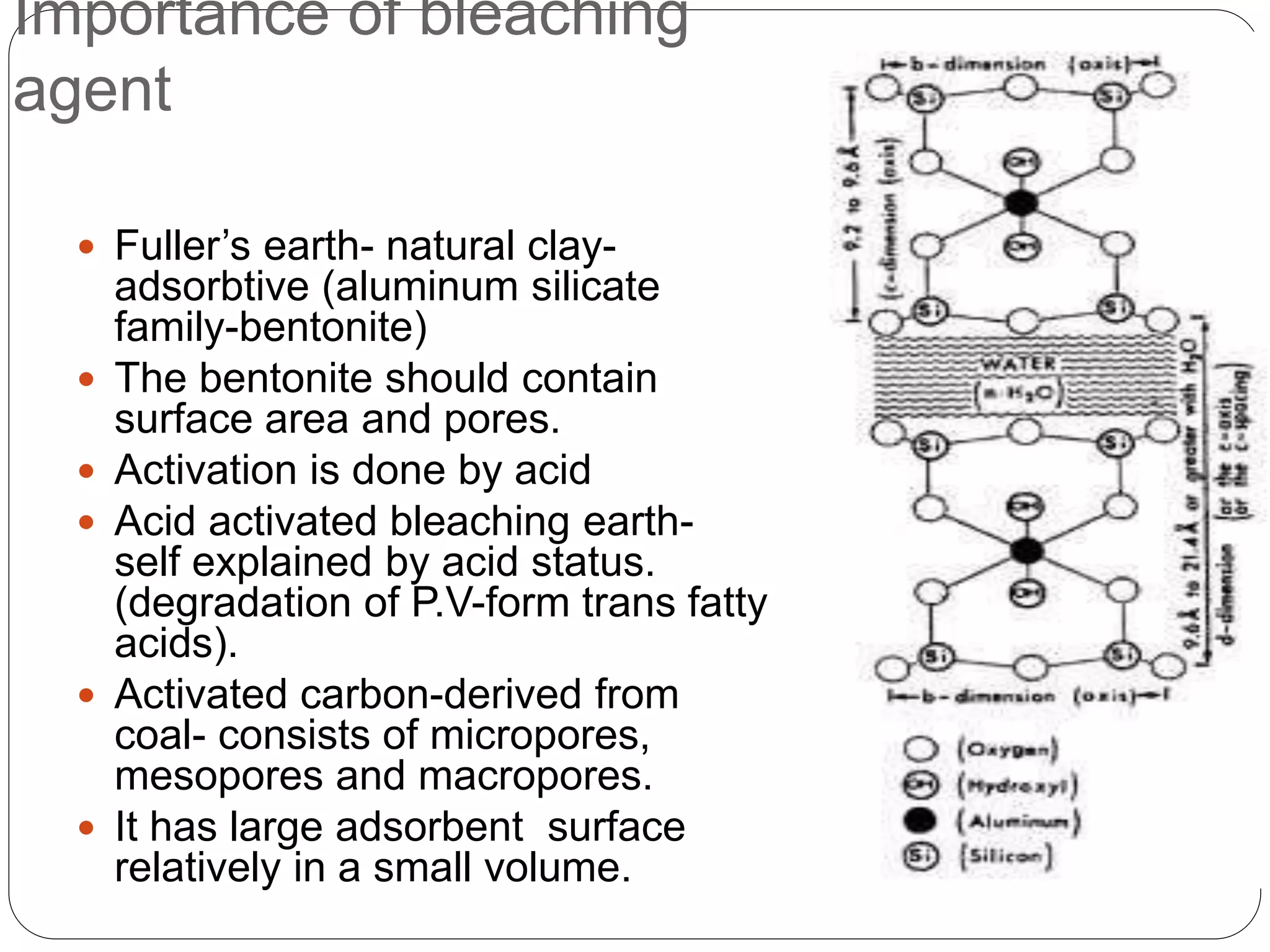
This document discusses oil and fat processing, specifically refining of oils and fats. It describes the major steps in refining which include lecithin removal, degumming, neutralization, bleaching, and deodorization. The purpose of refining is to remove undesirable components from crude oils and produce an edible oil with desirable characteristics like clear appearance and stability. Key steps include degumming to remove phospholipids, neutralization to remove free fatty acids, bleaching to remove color, and deodorization to remove odors and flavors through steam distillation under vacuum. Proper processing refines crude oils into finished oils suitable for human consumption.