Embed presentation
Download to read offline






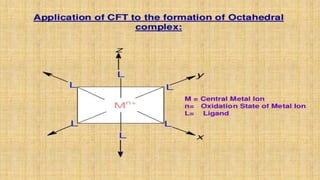
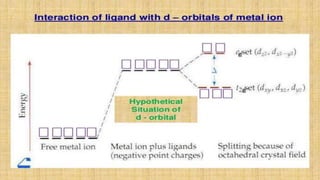
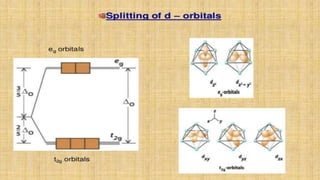

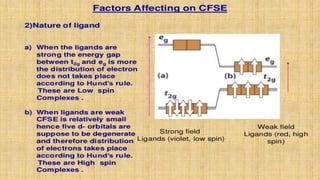



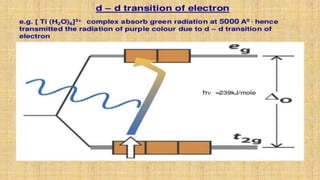
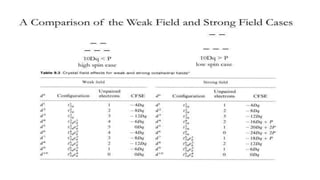



This document provides an overview of ligand field theory and its limitations. It contains an introduction to ligand field theory, a list of topics to be covered including assumptions, interactions between ligands and d-orbitals, factors that influence these interactions, applications, d-d transitions, tables of wavelengths and spin factors, and limitations. The limitations discussed are that ligand field theory ignores attractive forces between metal d-electrons and ligand nuclei, partial covalency of metal-ligand bonds, other metal orbitals besides d-orbitals, π-orbitals of ligands, and cannot explain relative ligand strengths or intensities of absorption bands.


















