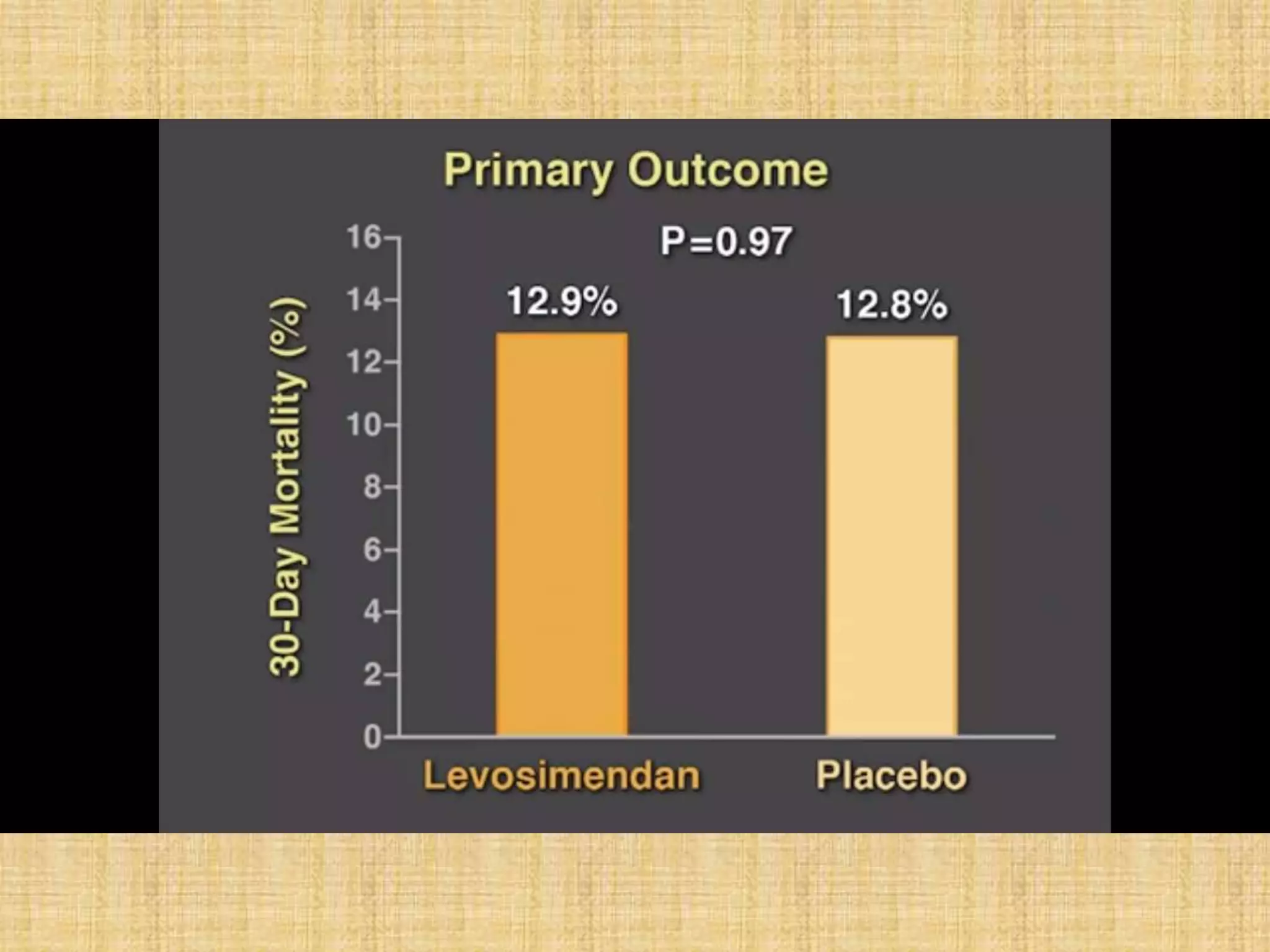
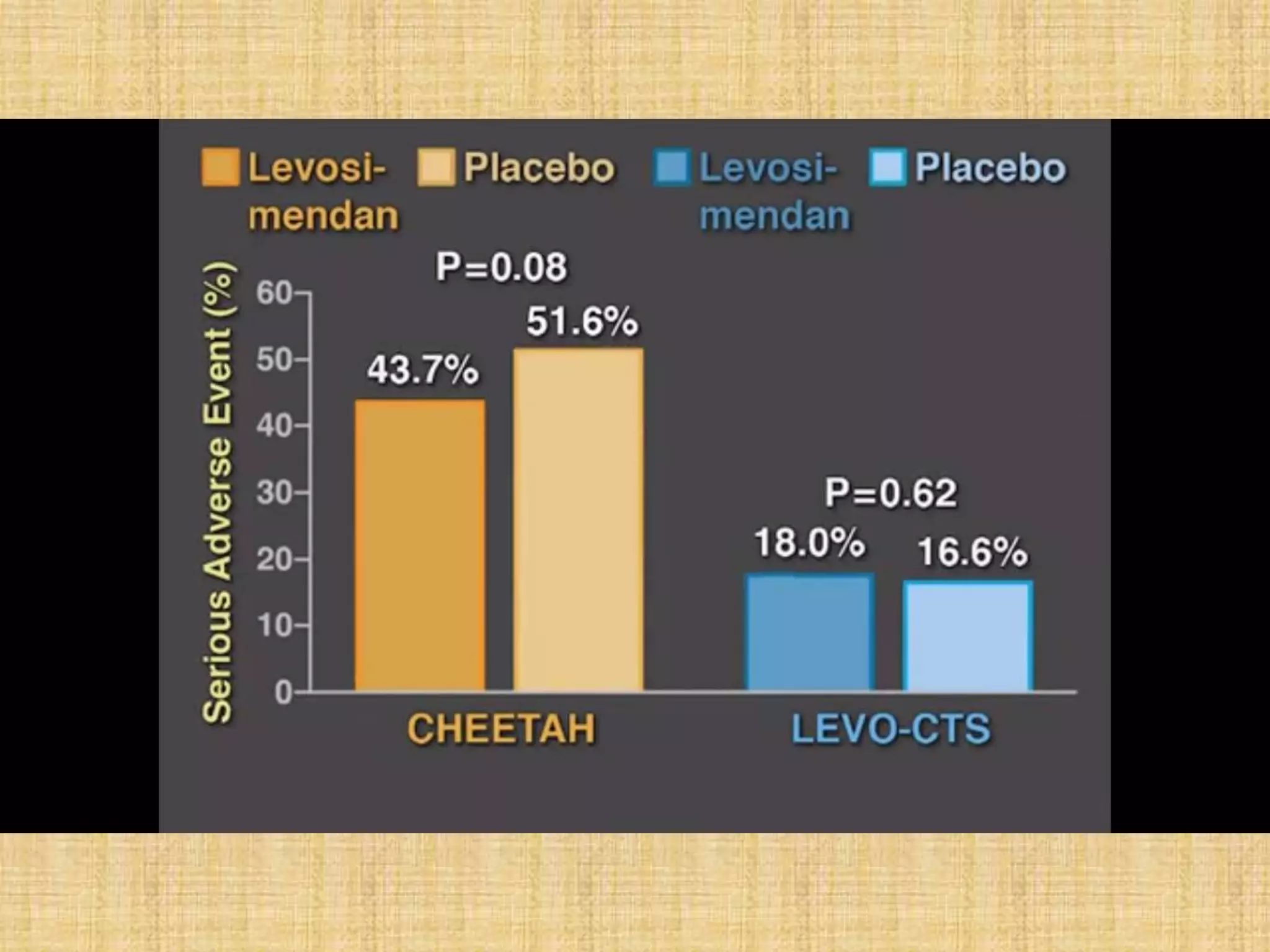
This document provides information on levosimendan, a calcium sensitizer used in the management of congestive heart failure. It discusses levosimendan's mechanism of action of enhancing cardiac contractility without increasing intracellular calcium levels. This reduces the drug's potential for arrhythmias compared to other inotropes. The document summarizes levosimendan's pharmacokinetics, dosing, efficacy demonstrated in clinical trials compared to dobutamine and placebo, and side effect profile. It positions levosimendan as an ideal agent for increasing cardiac output while minimizing oxygen demand, tachyphylaxis and arrhythmogenic potential.




![Inotropic drugs
Gs Gi
β-receptor
ATP cAMP (active)
rise in intracellular
calcium
Dobutamine
AMP (inactive)
PDE
Na+/Ca2+
exchanger
Na+/K+exchanger
[Ca2+]
[Na+]
[K+]
Digoxin
Milrinone
5](https://image.slidesharecdn.com/levosi-170607135927/75/Levosimendan-5-2048.jpg)