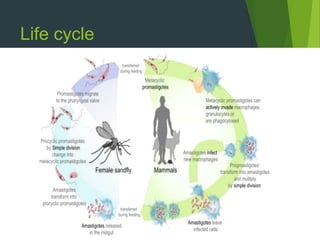
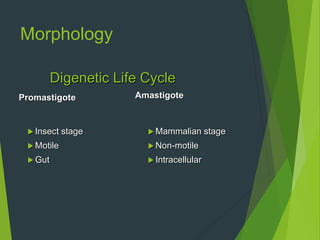
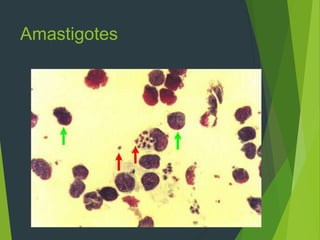
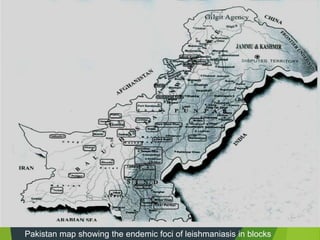
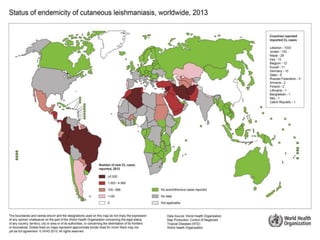
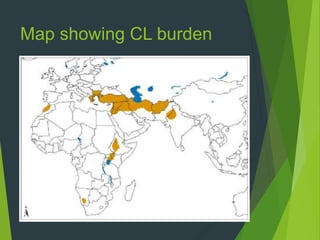
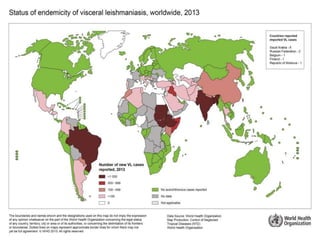
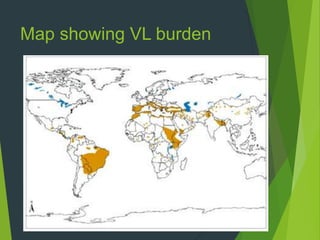
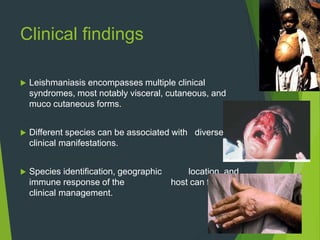
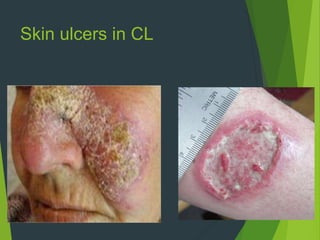
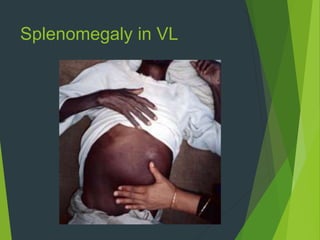
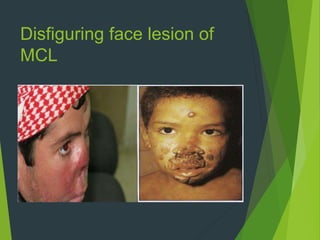
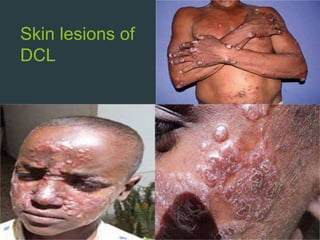
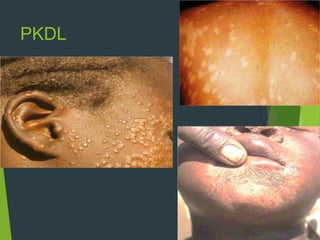
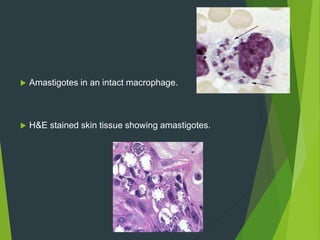
Leishmaniasis is a vector-borne disease caused by protozoan parasites from over 20 species of Leishmania, presenting in forms such as cutaneous, visceral, and muco-cutaneous leishmaniasis. The disease is transmitted primarily through bites from infected female sand flies, with differing transmission dynamics influenced by ecological settings, population mobility, and socioeconomic factors. Epidemiologically, leishmaniasis affects millions in developing regions, particularly in tropical and subtropical areas, with significant public health concerns stemming from cases in countries like India, Brazil, and Ethiopia.