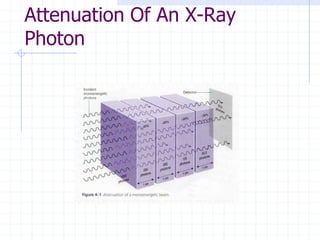
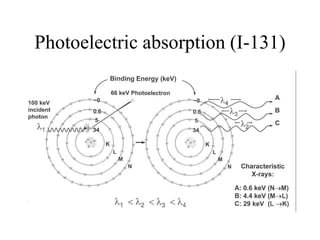
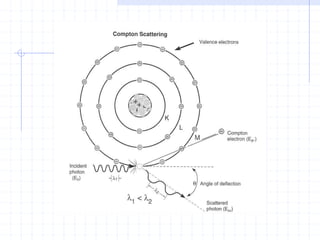
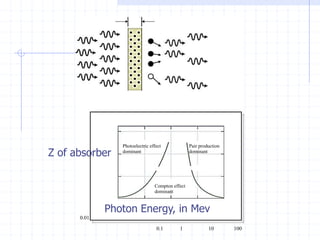
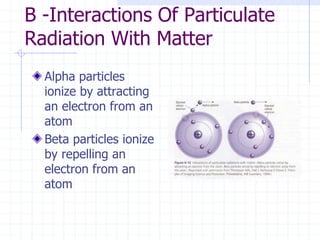
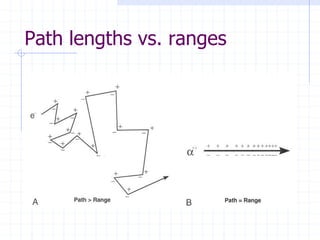
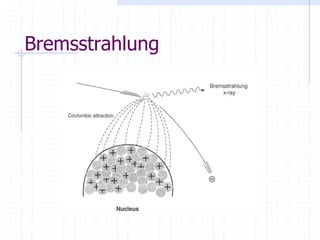
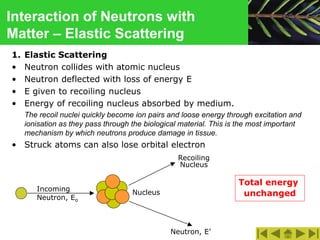
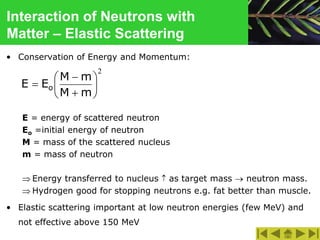
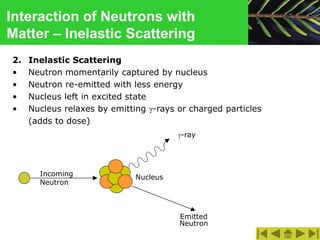
This document discusses the interactions of ionizing radiation with matter. It begins by introducing the objectives of understanding how radiation interacts with matter and the effects on physical, chemical, and biological levels. It then covers the three main interactions of photons (photoelectric effect, Compton scattering, pair production) and how they lead to attenuation. Key points include how probability depends on photon energy and atomic number, and the byproducts of each interaction. It also discusses the interactions of particulate radiation like electrons, protons, neutrons, and how they ionize matter through excitation, ionization, and bremsstrahlung. Factors like linear energy transfer and specific ionization are addressed. The document is intended to provide background knowledge on radiation detection and