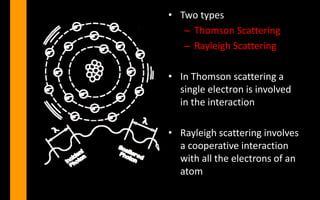
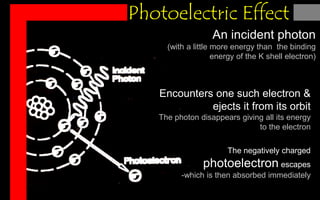
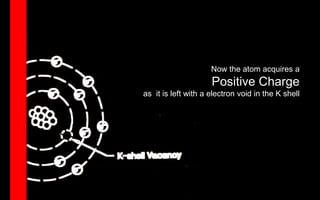
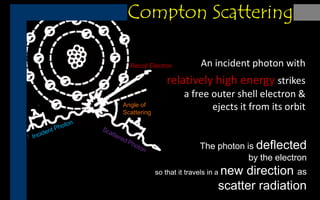
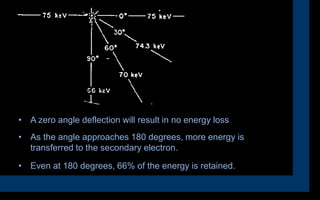
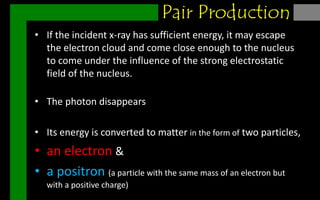
X-rays can interact with matter through various interactions such as coherent scattering, photoelectric effect, Compton scattering, pair production, and photodisintegration. The photoelectric effect and Compton scattering are the most important interactions for diagnostic x-rays. The photoelectric effect accounts for about 75% of interactions and results in the emission of characteristic x-rays. Compton scattering accounts for about 20% of interactions and scatters x-rays without energy loss, producing scatter radiation. The interaction that occurs depends on the photon energy and the atomic number of the absorbing material.