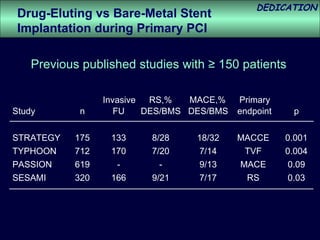
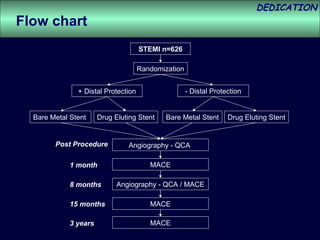
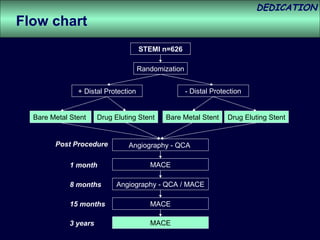
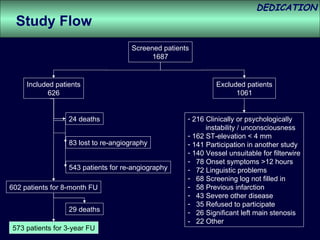
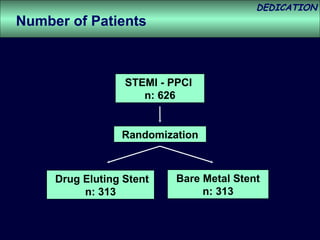
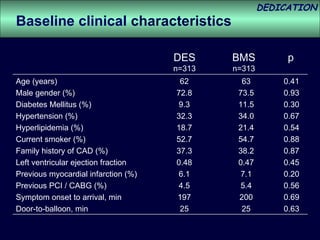
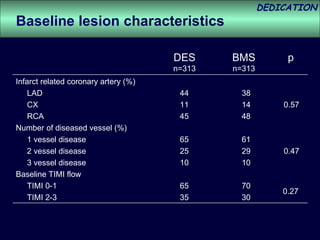
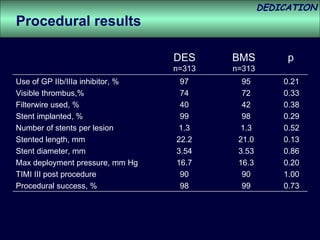
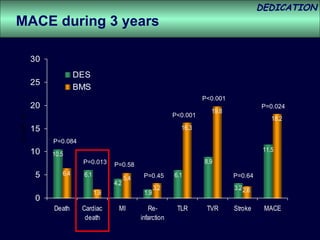
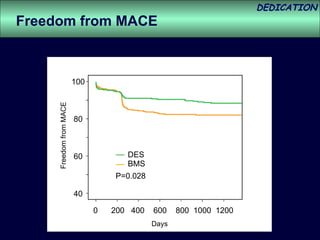
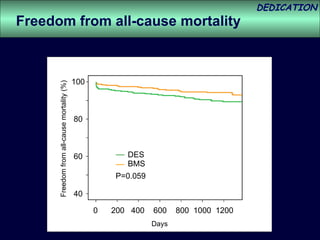
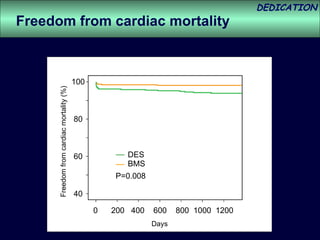
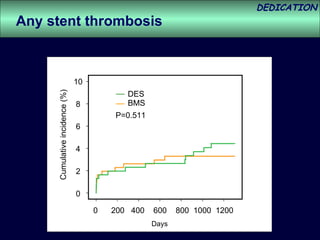
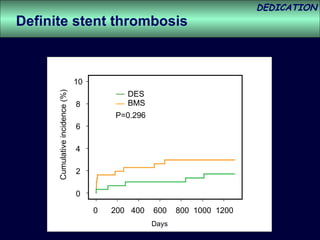
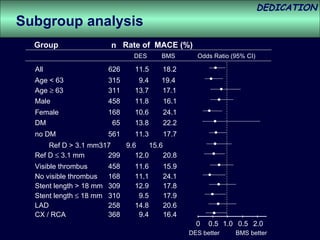
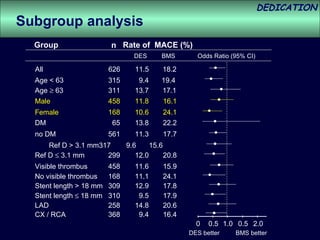
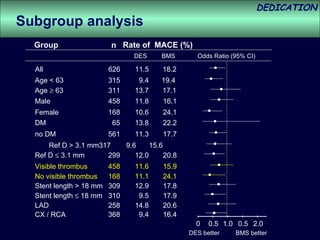
- The study evaluated clinical outcomes in 626 STEMI patients treated with primary percutaneous coronary intervention (PCI) who were randomized to receive either drug-eluting stents (DES) or bare-metal stents (BMS).
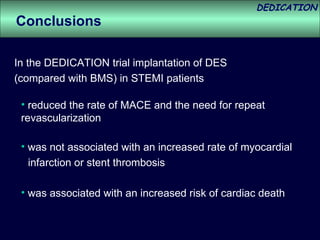
- At 3-year follow-up, patients who received DES had lower rates of major adverse cardiac events (MACE) and repeat revascularization compared to those receiving BMS, but no difference in myocardial infarction or stent thrombosis rates.
- DES implantation was associated with a higher risk of cardiac death compared to BMS.