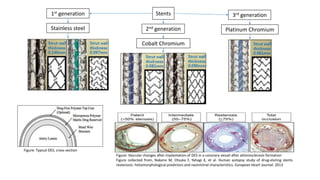
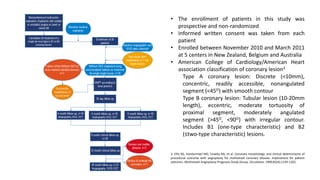
This study evaluated the MiStent, a bioabsorbable polymer-coated sirolimus-eluting stent. 30 patients received the MiStent and were followed up to 18 months. The primary outcome was in-stent late lumen loss (LLL), measured by angiography. LLL was low at 0.1 ± 0.2 mm at 8 months, with no evidence of increased loss later. Secondary outcomes included procedural success rates and clinical event rates. Preliminary safety and efficacy was observed up to 18 months, with crystalline sirolimus delivered locally up to 6 months without an initial burst, as seen with durable polymer stents. The MiStent demonstrated low LLL and neointimal obstruction with no binary rest