This document describes the BioFreedom trial, a prospective randomized trial comparing polymer-free Biolimus A9-eluting stents to paclitaxel-eluting stents. The trial found that the polymer-free stents were non-inferior to the paclitaxel stents in preventing late lumen loss at 12 months, meeting the primary endpoint. Late lumen loss was also lower for the polymer-free stents compared to the paclitaxel stents at 12 months. Both stent types showed sustained safety up to 12 months with no stent thromboses. The results provide initial evidence that polymer-free drug coated stents can inhibit neointimal hyperplasia as effectively
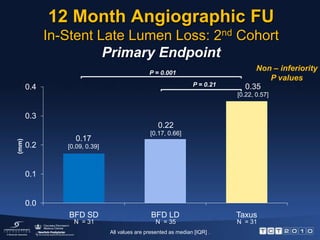
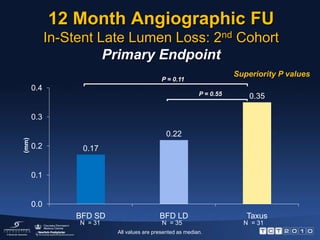
![12 Month Angiographic FUIn-Stent Late Lumen Loss: 2nd CohortPrimary EndpointNon – inferiority P valuesP = 0.001P = 0.21 (mm)N = 31 N = 31N = 35All values are presented as median [IQR] .](https://image.slidesharecdn.com/biofreedomtct10pressfinal-100927081349-phpapp02/85/Biofreedom-tct10-press-final-7-320.jpg)