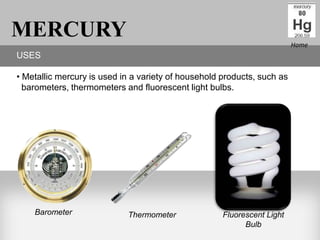
This presentation discusses several harmful elements from the periodic table, including their uses, effects on humans, and effects on the environment. It provides detailed information about beryllium, mercury, cobalt, lead, radon, nickel, and uranium - describing their common uses, how they impact human health, and how they can damage the environment. The document aims to educate readers about the dangers these elements can pose.