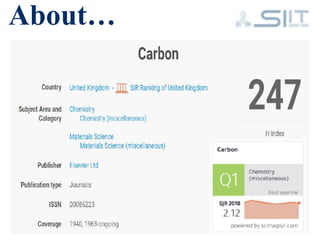
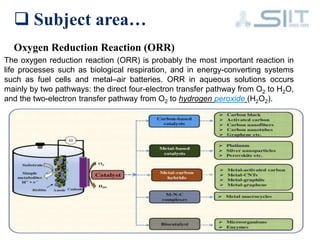
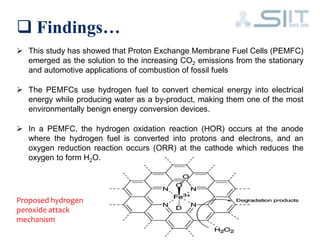
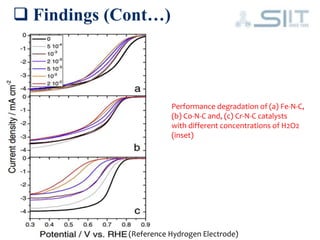
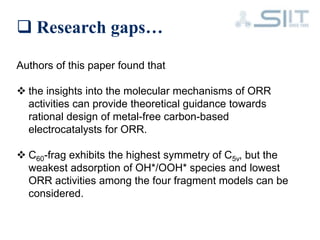
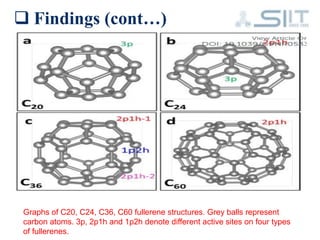
The document discusses carbon-based catalysts for oxygen reduction reaction (ORR). It summarizes three journal papers on this topic. The first paper discusses the need for non-precious metal catalysts to replace platinum in fuel cells and issues with stability and durability. The second paper examines nitrogen and metal-containing catalysts and their activity. The third paper analyzes ORR activity on different fullerene molecules and active sites. Overall, the document reviews research gaps and findings on developing durable, non-precious metal catalysts for ORR.