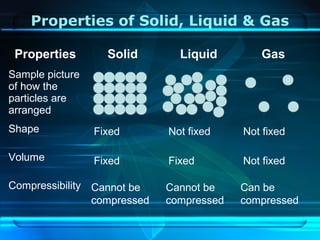
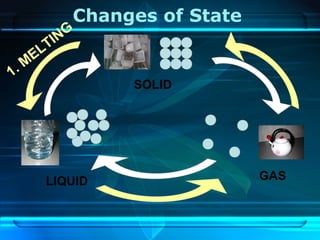
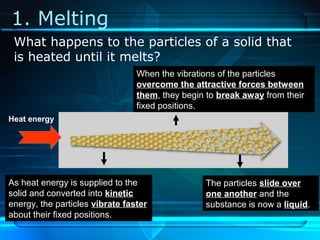
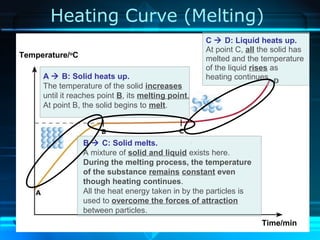
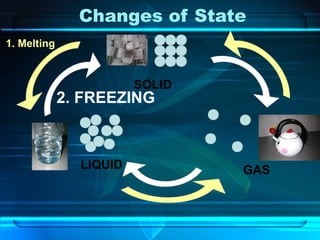
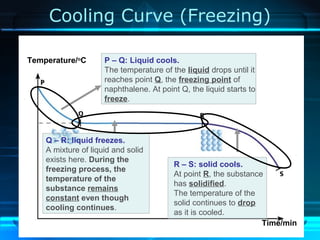
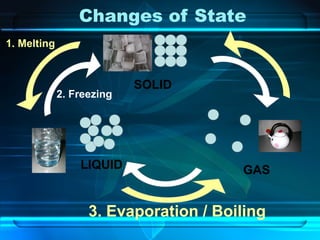
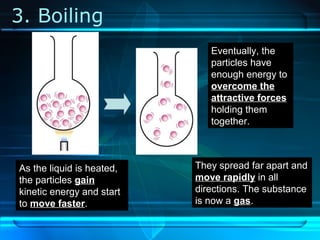
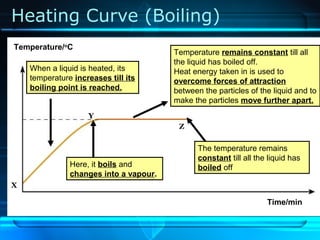
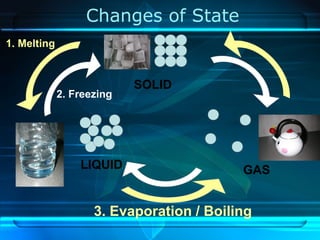
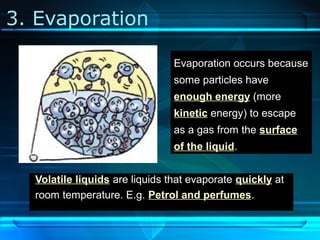
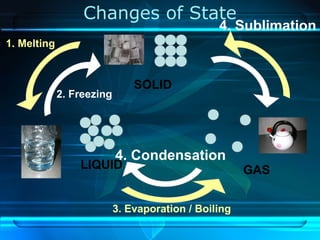
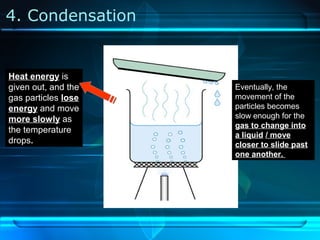
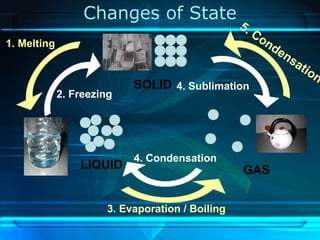
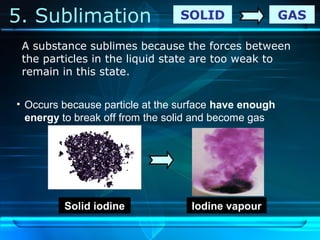
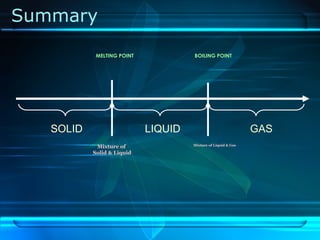
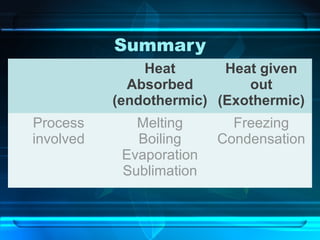
Kinetic particle theory states that all matter is made up of tiny particles in constant, random motion. The rate of particle vibration determines the state of matter. As heat is added, particles vibrate faster and can change the state from solid to liquid to gas. The reverse processes of freezing and condensation occur as heat is removed and particles slow down. Changes of state are reversible phase transitions that occur at characteristic melting and boiling points as heat is absorbed or released during the transition.