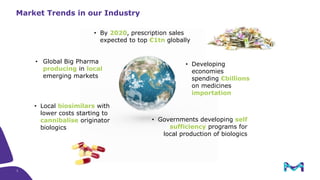
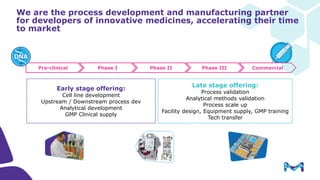
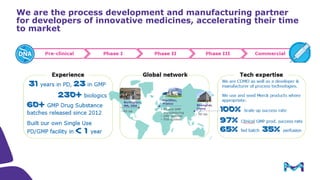
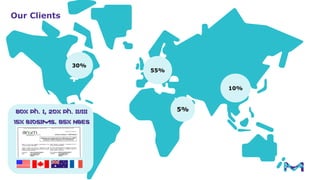
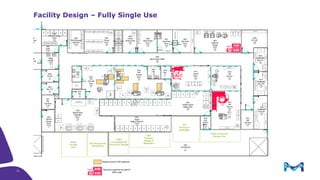
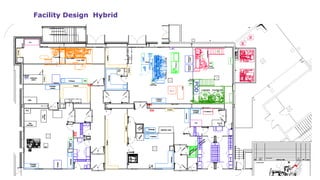
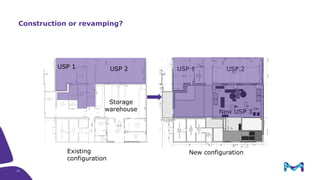
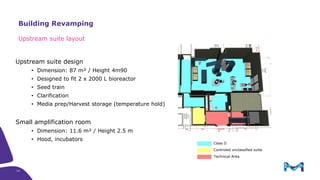
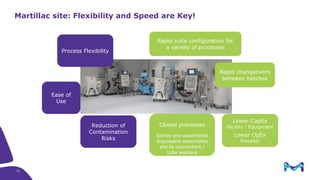
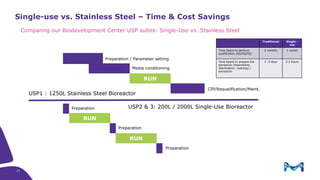
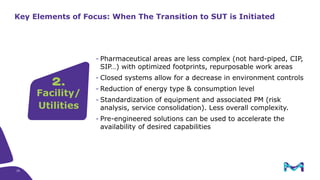
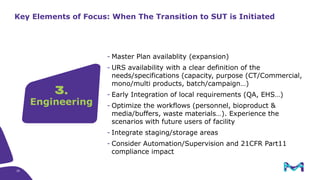
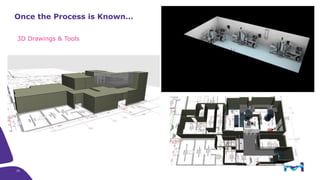
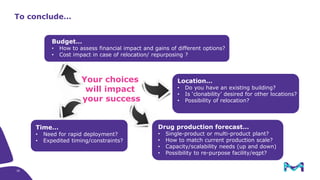
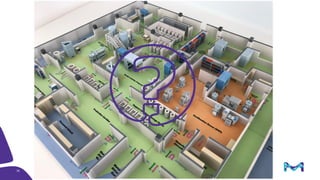
The document outlines key considerations for building a cGMP biomanufacturing facility, emphasizing the importance of flexibility, process design, and regulatory compliance. It discusses the market trends in biomanufacturing, the design and engineering requirements, and the benefits of single-use technologies over traditional methods. Additionally, it highlights the project's planning phases and the need for a robust project management approach to ensure operational efficiency and cost-effectiveness.