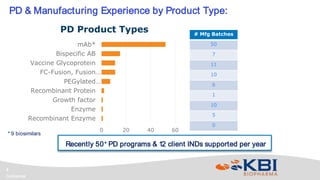
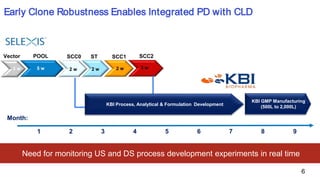
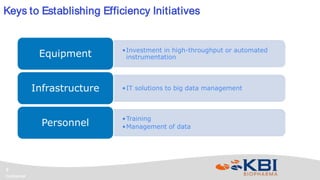
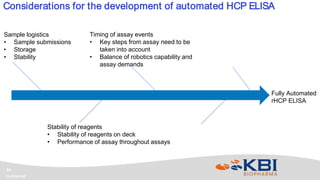
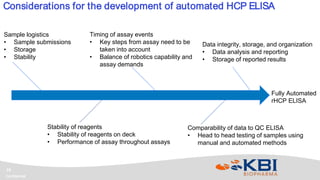
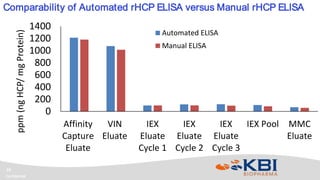
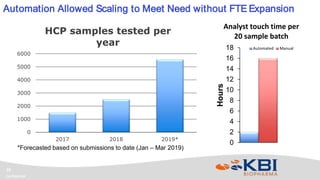
KBI Biopharma has developed high-throughput and automated processes to accelerate biotherapeutic development. This includes establishing a high-throughput process development team utilizing automated equipment and informatics solutions. Analytical case studies demonstrate automation of a residual host cell protein ELISA using a liquid handling robot, reducing analysis time from hours to minutes per sample. A second case study outlines development of a high-throughput size exclusion chromatography method, reducing run time from 30 minutes to 6 minutes while still effectively screening for high molecular weight species. These efforts allow for real-time data generation and monitoring of process development experiments.