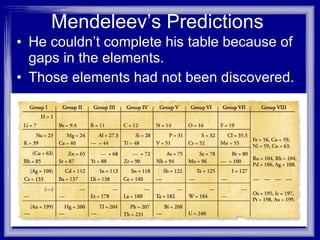
1) Mendeleev arranged the elements in his periodic table based on increasing atomic mass, with elements of similar properties placed in columns.
2) His table had gaps for elements not yet discovered, but he predicted their properties. When these were later discovered, such as gallium, they matched his predictions, verifying his periodic table.
3) The periodic table is organized so that elements are classified based on atomic structure, with properties repeating periodically from row to row.