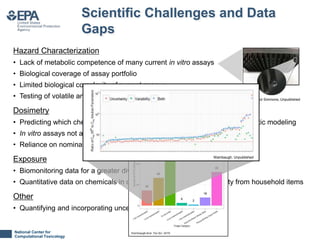
This document summarizes progress made and remaining challenges in developing new approach methods for regulatory toxicology assessments. It outlines several new approach methods developed including high-throughput in vitro screening databases and models. However, it notes remaining scientific challenges including limited metabolic and biological complexity of current in vitro assays and gaps in exposure and dosimetry data. The document also discusses philosophical challenges in validating new approach methods, defining adversity at the molecular level, and gaining acceptance for qualitative and quantitative uncertainties compared to traditional animal studies.