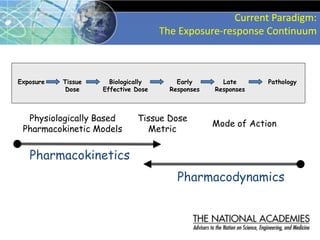
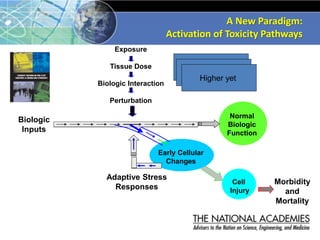
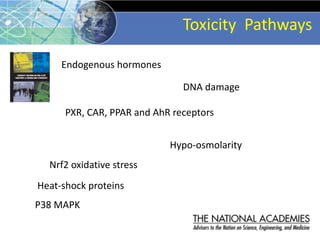
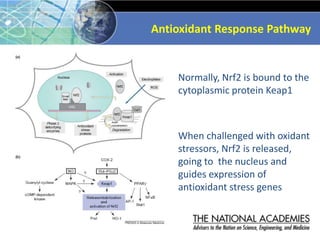
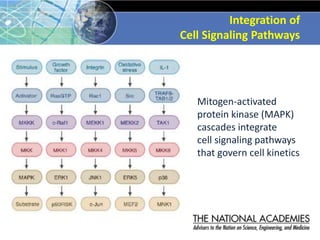
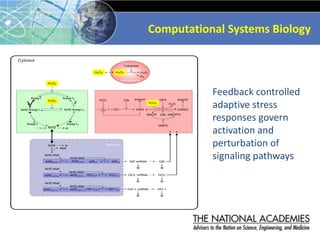
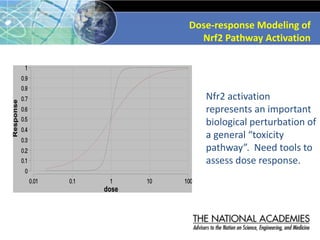
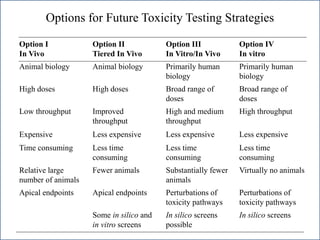
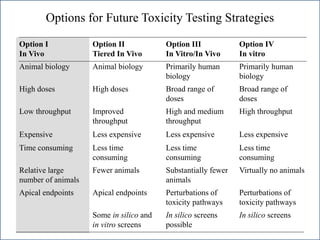
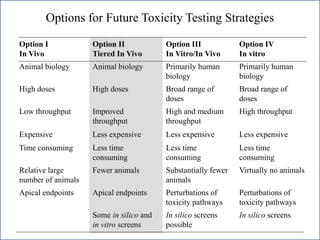
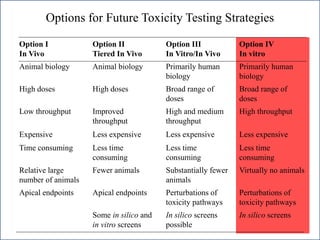
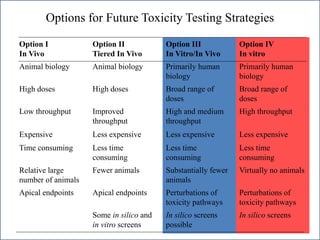
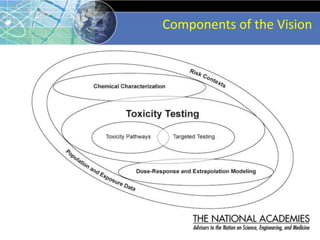
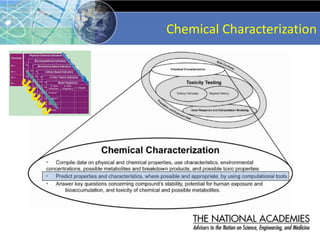
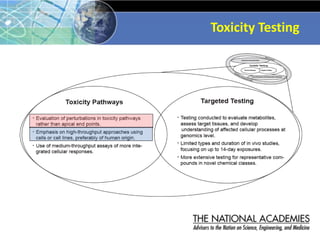
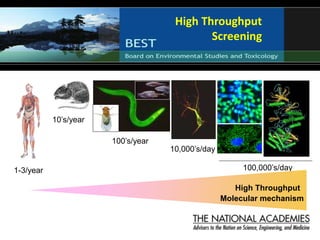
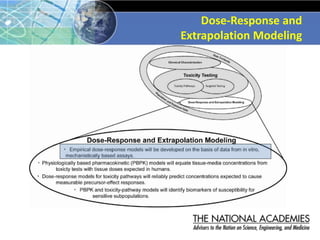
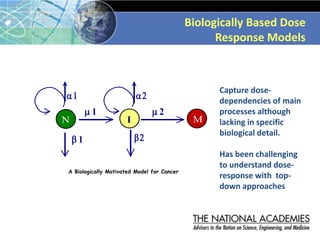
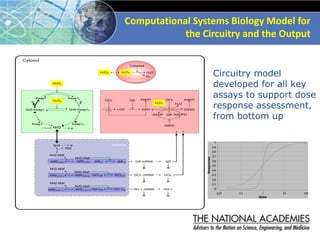
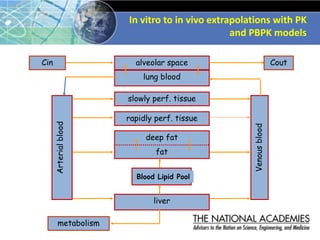
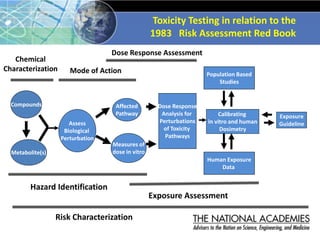
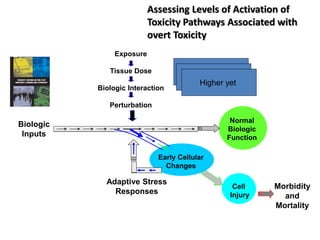
The document outlines a strategy and vision for modernizing toxicity testing in the 21st century. It proposes moving away from traditional animal testing approaches and instead focusing on assessing perturbations of toxicity pathways in human cells through high-throughput in vitro screening. This would allow testing a much broader range of chemicals at lower costs and with fewer animals. Computational modeling of dose-response relationships would be used to extrapolate in vitro results for risk assessment purposes. The approach has implications for toxicity testing practices and regulations. Overall, the vision is to create a new paradigm centered on human pathways rather than apical animal outcomes.