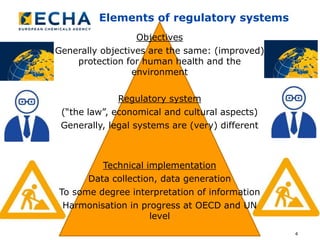
The document discusses the prioritization of chemicals within the EU framework, focusing on the regulatory risk management measures under REACH and CLP regulations. It emphasizes the importance of harmonization in data collection, sharing, and analysis to improve human health and environmental protection. The text also highlights ongoing collaboration efforts among international authorities to enhance exposure data usage and streamline assessment processes.