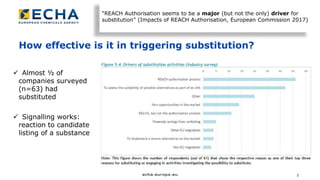
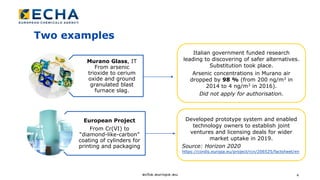
The document discusses the effectiveness of the REACH authorisation system in improving the management of substances of very high concern (SVHCs), indicating that nearly half of surveyed companies have substituted dangerous substances. It highlights that while the system has led to significant risk reductions and safer practices, it faces challenges such as high costs, ambiguous legal texts, and varied perceptions among stakeholders. Overall, the authorisation system has shown potential for substitution but requires improvements in efficiency and predictability to better serve both applicants and society.