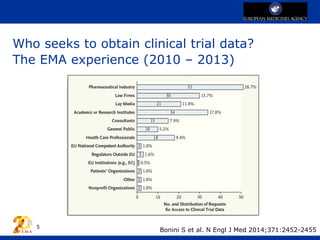
The European Medicines Agency (EMA) has implemented a policy for sharing clinical trial data to balance stakeholder interests, effective from January 1, 2015. This initiative is aimed at fostering transparency, improving trial design, and informing future research, while ensuring patient anonymity and data confidentiality. The EMA's experience thus far has encouraged pharmaceutical companies to proactively participate in data sharing and shifted the discourse from confidentiality concerns to constructive collaboration.


![Date of coming into effect of policy
1/1/2015 Any new Marketing Authorisation Applications […],
submitted after this date
1/7/2015 Extension of indication applications and line extension
applications for existing products, submitted after this
date
3
First phase: Clinical study reports
Second phase: Individual patient data (IPD) sharing
modalities to be discussed
Not in scope: Legacy data (submitted before
01/01/2015) [or non-centrally authorised products].](https://image.slidesharecdn.com/1601075eichler-161012151018/85/Opening-clinical-trial-data-4-320.jpg)