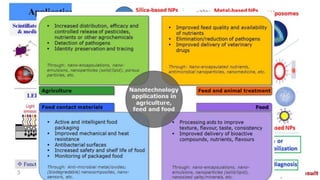
The debate, moderated by Roger Drew, discusses whether product stewardship can effectively replace regulation for the safe management of nanomaterials (NMs). Key questions raised include the robustness of chemical legislation for NMs, the industry's role in product stewardship, and the need for explicit testing requirements to ensure safety for workers and consumers. Both debaters, David Azoulay and David Warheit, explore the advantages and challenges of each approach in ensuring the safe use of nanomaterials.




![Questions from the forum organiser:
• Is chemical legislation robust enough for NM’s?
• What’s the role of industry product stewardship?
• How can companies show due diligence?
6
• Can workers and consumers [researchers] have
confidence in safe use of NM, or should there be
explicit testing requirements?](https://image.slidesharecdn.com/1rogerdrewmoderatordebate15-180618151853/85/HCF-2018-Depate-introduction-Roger-Drew-6-320.jpg)
