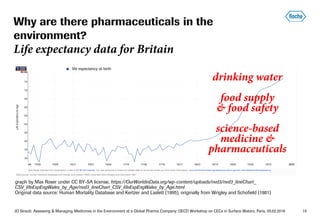
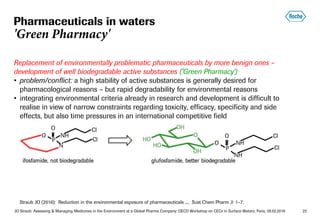
This document summarizes Jürg Oliver Straub's presentation on assessing and managing medicines in the environment at Roche, a global pharmaceutical company. Some key points:
- Roche is a large Swiss pharmaceutical company with production sites worldwide and many well-known drugs.
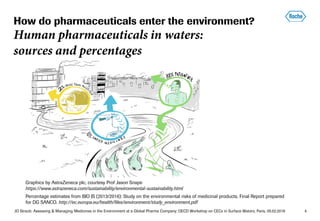
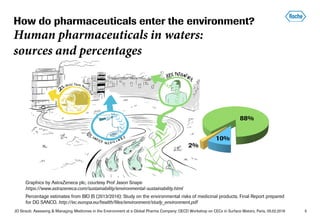
- Most pharmaceuticals enter the environment through human use from improper disposal down sinks/toilets and excretion. Roche works to reduce this through take-back programs and increasing awareness.
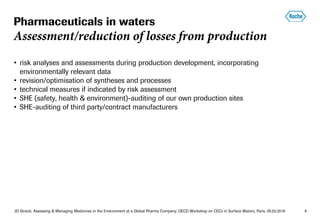
- Roche incorporates environmental risk assessments into drug development and production to minimize release of pharmaceuticals into wastewater. They audit their own and third-party production sites.
- Beyond development, Roche conducts environmental risk assessments, supports industry stewardship programs,


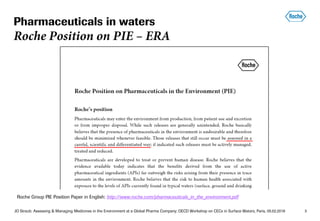





![JO Straub: Assessing & Managing Medicines in the Environment at a Global Pharma Company; OECD Workshop on CECs in Surface Waters, Paris, 05.02.2018 12
• European human pharmaceuticals industry task force on PIE, comprising AESGP
(self-medication), EFPIA (research pharma) and Medicines for Europe (generics &
biosimilars), since 2012
• EcoPharmacoStewardship (EPS) life-cycle approach, programme with four pillars:
Pharmaceuticals in waters
Industry co-operation on Pharmaceuticals in the
Environment (PIE)
Effluent Management
assessing & managing
production effluents,
wastewater maturity
ladder concept
[Caldwell et al. 2016]
eERA
extended environmental
risk assessment
concept for
pharmaceuticals, going
beyond patent duration
Outreach
awareness campaign
for correct disposal of
unused medicines
in Europe:
www.medsdisposal.eu
#medsdisposal
iPiE
€10m EU/EFPIA
research project on
intelligent assessment
and prioritisation of
pharmaceuticals in the
environment: i-pie.org
Caldwell DJ et al. (2016): A risk-based approach to managing active pharmaceutical ingredients in manufacturing effluent. Environ Toxicol Chem 35: 813–822.
www.aesgp.eu www.efpia.eu www.medicinesforeurope.com](https://image.slidesharecdn.com/session3-jurgolivierstraub-180207162128/85/Session-3-Jurg-Oliver-Straub-12-320.jpg)