1 ch3 student to complete their_notes_12_13_
•Download as PPT, PDF•
1 like•368 views
JJ Thomson discovered the electron through his work with cathode ray tubes in the late 1800s. This led him to propose the plum pudding model of the atom, with negatively charged electrons embedded in a positively charged medium. Robert Millikan later directly measured the charge of individual electrons through his oil drop experiment in 1905, enabling him to calculate the electron's mass. Ernest Rutherford then used the gold foil experiment to discover the nucleus as the small, dense, positively charged center of atoms through deflections of alpha particles in 1910.
Report
Share
Report
Share
Recommended
1 ch3 student to complete their_notes_12_13_

J.J. Thomson discovered the electron through experiments with cathode ray tubes. He found the mass to charge ratio of electrons and proposed the plum pudding model of the atom, where electrons are embedded in a positively charged material. Robert Millikan performed the oil drop experiment, which measured the minimum electric charge and enabled finding the mass of the electron. Ernest Rutherford conducted the gold foil experiment, which demonstrated that atoms have a small, dense, positively charged nucleus.
Famous scientist who contributed to structure of atom!

This document provides biographical information about the English chemist and physicist John Dalton. It describes that Dalton was born in 1766 in Cumberland, England into a Quaker family. As a young man, he helped his brother run a Quaker school. He was influenced by his friend Elihu Robinson, an instrument maker and meteorologist, which sparked his interests in mathematics and meteorology. The document outlines Dalton's contributions including his research on color blindness, for which the term "Daltonism" was coined, as well as his work on the reflection and refraction of light. It describes Dalton's atomic theory which proposed that elements are made of atoms and that atoms of different elements have different weights. The document also notes some
Michael Faraday ppt

Michael Faraday was a British scientist born in 1791 who made seminal contributions to electromagnetism and electrochemistry. Though he had little formal education, he educated himself by attending lectures and taking detailed notes. He later worked as an assistant to Humphry Davy and conducted experiments that established electromagnetic induction and the principles of electricity generation. Some of Faraday's key discoveries included the electric motor, generators, and the foundations of electromagnetism. His innovative work and concepts helped enable the development of modern electromagnetic technology.
Thomson slide

J.J. Thomson discovered the electron in 1897 through experiments with cathode rays in a tube in England. He found that atoms are composed of electrons distributed throughout, contributing to atomic theory. His model pictured the atom as a plum pudding with electrons embedded inside, though he called them corpuscles.
Thomson slide

J.J. Thomson discovered the electron in 1897 through experiments with cathode rays in a tube. He found that atoms are composed of electrons distributed throughout, contributing to atomic theory. Thomson was born in 1856 in England and taught at Rotherfort, developing the "plum pudding" model where electrons are embedded in a positive charge, though he referred to them as "corpuscles".
Michael Faraday

Michael Faraday was a British scientist who made significant contributions to the fields of electromagnetism and electrochemistry. Some of his key accomplishments included discovering electromagnetic induction and inventing the electric motor. He was born in 1791 and spent his early life apprenticed as a bookbinder. In 1812, he was introduced to scientist Humphry Davy, who mentored Faraday and hired him as a laboratory assistant at the Royal Institution. Over the following decades, Faraday published numerous influential works and made pioneering discoveries that established the foundations of electromagnetism as a major science. He spent his later life conducting research, lecturing, and advising various scientific organizations until his death in 1867.
Discovery of neutron 

Sir James Chadwick discovered the neutron in 1932. He was awarded the 1935 Nobel Prize in Physics for this discovery. Chadwick performed an experiment where he smashed alpha particles into beryllium and allowed the radiation to hit paraffin wax. The results showed collisions with beryllium atoms released massive neutral particles, which Chadwick named neutrons. Neutrons have the same mass as protons but have no charge. Chadwick's discovery of the neutron helped reveal properties of atomic nuclei.
James chadwick

James Chadwick was a British physicist born in 1891 who discovered the neutron. He studied at the University of Manchester and University of Cambridge, and later became a professor of physics at the University of Liverpool in 1935. Through experiments, he verified that uncharged particles about the mass of protons existed in atoms, which he named neutrons in 1932.
Recommended
1 ch3 student to complete their_notes_12_13_

J.J. Thomson discovered the electron through experiments with cathode ray tubes. He found the mass to charge ratio of electrons and proposed the plum pudding model of the atom, where electrons are embedded in a positively charged material. Robert Millikan performed the oil drop experiment, which measured the minimum electric charge and enabled finding the mass of the electron. Ernest Rutherford conducted the gold foil experiment, which demonstrated that atoms have a small, dense, positively charged nucleus.
Famous scientist who contributed to structure of atom!

This document provides biographical information about the English chemist and physicist John Dalton. It describes that Dalton was born in 1766 in Cumberland, England into a Quaker family. As a young man, he helped his brother run a Quaker school. He was influenced by his friend Elihu Robinson, an instrument maker and meteorologist, which sparked his interests in mathematics and meteorology. The document outlines Dalton's contributions including his research on color blindness, for which the term "Daltonism" was coined, as well as his work on the reflection and refraction of light. It describes Dalton's atomic theory which proposed that elements are made of atoms and that atoms of different elements have different weights. The document also notes some
Michael Faraday ppt

Michael Faraday was a British scientist born in 1791 who made seminal contributions to electromagnetism and electrochemistry. Though he had little formal education, he educated himself by attending lectures and taking detailed notes. He later worked as an assistant to Humphry Davy and conducted experiments that established electromagnetic induction and the principles of electricity generation. Some of Faraday's key discoveries included the electric motor, generators, and the foundations of electromagnetism. His innovative work and concepts helped enable the development of modern electromagnetic technology.
Thomson slide

J.J. Thomson discovered the electron in 1897 through experiments with cathode rays in a tube in England. He found that atoms are composed of electrons distributed throughout, contributing to atomic theory. His model pictured the atom as a plum pudding with electrons embedded inside, though he called them corpuscles.
Thomson slide

J.J. Thomson discovered the electron in 1897 through experiments with cathode rays in a tube. He found that atoms are composed of electrons distributed throughout, contributing to atomic theory. Thomson was born in 1856 in England and taught at Rotherfort, developing the "plum pudding" model where electrons are embedded in a positive charge, though he referred to them as "corpuscles".
Michael Faraday

Michael Faraday was a British scientist who made significant contributions to the fields of electromagnetism and electrochemistry. Some of his key accomplishments included discovering electromagnetic induction and inventing the electric motor. He was born in 1791 and spent his early life apprenticed as a bookbinder. In 1812, he was introduced to scientist Humphry Davy, who mentored Faraday and hired him as a laboratory assistant at the Royal Institution. Over the following decades, Faraday published numerous influential works and made pioneering discoveries that established the foundations of electromagnetism as a major science. He spent his later life conducting research, lecturing, and advising various scientific organizations until his death in 1867.
Discovery of neutron 

Sir James Chadwick discovered the neutron in 1932. He was awarded the 1935 Nobel Prize in Physics for this discovery. Chadwick performed an experiment where he smashed alpha particles into beryllium and allowed the radiation to hit paraffin wax. The results showed collisions with beryllium atoms released massive neutral particles, which Chadwick named neutrons. Neutrons have the same mass as protons but have no charge. Chadwick's discovery of the neutron helped reveal properties of atomic nuclei.
James chadwick

James Chadwick was a British physicist born in 1891 who discovered the neutron. He studied at the University of Manchester and University of Cambridge, and later became a professor of physics at the University of Liverpool in 1935. Through experiments, he verified that uncharged particles about the mass of protons existed in atoms, which he named neutrons in 1932.
michael faraday

Michael Faraday was an English scientist who contributed significantly to the fields of electromagnetism and electrochemistry. Born into poverty in 1791, he received little formal education but became an assistant to scientist Humphry Davy. Through his own experimentation, Faraday discovered electromagnetic induction and invented the electric motor, laying the foundation for modern electricity. He also made important contributions to chemistry, discovering benzene and other aromatic compounds. Faraday introduced concepts of electromagnetic fields that became the basis of modern field theory in physics. He remained an influential scientist at the Royal Institution in London for over 50 years until his death in 1867.
James Chadwick

James Chadwick was a British physicist born in England. He studied under Ernest Rutherford and worked with him on experiments involving radioactive substances. This led Chadwick to continue Rutherford's idea that there were neutral particles in the nucleus of an atom. Through his own experiment in 1932, Chadwick was able to prove the existence of the neutron. He called these neutral particles "neutrons" and showed they have about the same mass as a proton but no electric charge. This discovery of the neutron was fundamental to modern physics and earned Chadwick the 1935 Nobel Prize in Physics.
Michael faraday powerpoint

Michael Faraday was born in 1791 in London to a poor family. He had little formal education but showed an interest in science through his work as a bookbinder. He became an assistant to scientist Humphry Davy at the Royal Institution, where he began his research into electricity and magnetism. Through experiments with coils and iron rings, he discovered the principles of electromagnetic induction and how changing magnetic fields can generate electric currents. His invention of the electric generator in 1831, which used a copper disk between magnet poles to produce a continuous electric current, allowed electricity to be efficiently produced and paved the way for widespread electrification.
Michael faraday

Michael Faraday was a British scientist who made many important contributions to the fields of
electromagnetism and electrochemistry in the 19th century. Through experiments such as discovering
electromagnetic induction and establishing the laws of electrolysis, Faraday laid the groundwork for modern
electric technology and helped scientists understand the relationship between electricity, magnetism, and
light. He published his findings in books and papers that communicated complex scientific concepts to
ordinary readers. Though born into poverty, Faraday rose to prominence through his work at the Royal
Institution of Great Britain, where he spent over 50 years conducting experiments and lectures.
Discovery of Electrons and Protons

This power point slides presents how the electrons and protons were discovered together with the personalities involved with this scientific breakthrough.
Michael Faraday

Michael Faraday was born in 1791 in England and had little formal education but became interested in science. He became an apprentice to scientist Humphry Davy, which launched his scientific career. Faraday made many important contributions including discovering benzene and two chlorine/carbon compounds. He invented the Bunsen burner and established the foundations of electromagnetism by producing the first electric motor and generator. Faraday published widely and popularized scientific terms and methods through lectures, making complex topics more accessible. He was honored with scientific medals and awards for his discoveries that still impact fields like chemistry and electricity today.
Michael faraday

Michael Faraday was an English scientist who made many important contributions to the fields of electromagnetism and electrochemistry. Though he received little formal education, through his own research he established the basis of the electromagnetic field and discovered the principles of electromagnetic induction and electrolysis. His inventions of electromagnetic devices formed the foundation of electric motor technology. He declined offers of knighthood and presidency of the Royal Society, preferring to remain "plain Mr. Faraday" throughout his life.
Faraday

Michael Faraday was a pioneering British scientist from a working class background who made seminal contributions to electromagnetism and electrochemistry through his patient, diligent experiments. His most influential inventions include the electric motor, generator and transformer. These breakthroughs helped establish the foundation of modern electricity and propelled society to new levels of technological development. Though honored with prestigious offers, Faraday remained humble and dedicated to scientific discovery throughout his life.
Ernest Rutherford

Ernest Rutherford conducted an experiment in 1911 where he fired positively charged alpha particles at a thin gold foil. Contrary to expectations, many particles were deflected or bounced back, showing that the atom's mass was concentrated in a small nucleus rather than evenly distributed as Thomson's plum pudding model predicted. This led Rutherford to propose the nuclear model of the atom, with electrons orbiting a dense central nucleus, revolutionizing the field of nuclear physics.
James chadwick

Chadwick discovered a new particle in the nucleus called the neutron through experiments bombarding various elements with alpha particles. The neutron was predicted by Majorana due to its lack of an electrical charge, allowing it to penetrate nuclei without overcoming electromagnetic repulsion like protons. Using Rutherford's zinc shield technique, Chadwick determined neutrons could enter even the heaviest element nuclei, helping establish the neutron as a key part of the nuclear model and defining the atom's mass.
MICHAEL FARADAY PPT..

life style of great scientist Michael Faraday .....!
Michael Faraday, who came from a very poor family, became one of the greatest scientists in history. His achievement was remarkable in a time when science was the preserve of people born into privileged families. The unit of electrical capacitance is named the farad in his honor, with the symbol F.
The faraday is a dimensionless unit of electric charge quantity, equal to approximately 6.02 x 10 23 electric charge carriers. This is equivalent to one mole , also known as Avogadro's constant .
Education and Early Life
Michael Faraday was born on September 22, 1791 in London, England, UK. He was the third child of James and Margaret Faraday. His father was a blacksmith who had poor health. Before marriage, his mother had been a servant. The family lived in a degree of poverty.
Michael Faraday attended a local school until he was 13, where he received a basic education. To earn money for the family he started working as a delivery boy for a bookshop. He worked hard and impressed his employer. After a year, he was promoted to become an apprentice bookbinder
Michael Faraday’s Scientific Achievements and Discoveries:
It would be easy fill a book with details of all of Faraday’s discoveries – in both chemistry and physics. It is not an accident that Albert Einstein used to keep photos of three scientists in his office: Isaac Newton, James Clerk Maxwell and Michael Faraday.
Funnily enough, although in Faraday’s lifetime people had started to use the word physicist, Faraday disliked the word and always described himself as a philosopher. 1821: Discovery of Electromagnetic Rotation
This is a glimpse of what would eventually develop into the electric motor, based on Hans Christian Oersted’s discovery that a wire carrying electric current has magnetic properties.
1823: Gas Liquefaction and Refrigeration
In 1802 John Dalton had stated his belief that all gases could be liquified by the use of low temperatures and/or high pressures. Faraday provided hard evidence for Dalton’s belief by applying pressure to liquefy chlorine gas and ammonia gas for the first time.
1825: Discovery of Benzene
Historically, benzene is one of the most important substances in chemistry, both in a practical sense – i.e. making new materials; and in a theoretical sense – i.e. understanding chemical bonding. Michael Faraday discovered benzene in the oily residue left behind from producing gas for lighting in London.
1831: Discovery of Electromagnetic Induction
Faraday discovered that a varying magnetic field causes electricity to flow in an electric circuit.
1834: Faraday’s Laws of Electrolysis
This is the science of understanding what happens at the interface of an electrode with an ionic substance. Electrochemistry is the science that has produced the Li ion batteries and metal hydride batteries capable of powering modern mobile technology. Faraday’s laws are vital to our understanding of electrode reactions.
discovery of proton 

Eugen Goldstein was a German physicist born in 1850 who made early investigations of discharge tubes and discovered anode rays. He is sometimes credited with the discovery of the proton. In the 1870s, Goldstein undertook his own investigations of discharge tubes and named the light emissions studied by others cathode rays. Goldstein conducted experiments with canal rays that contributed to the discovery of the proton.
Structure of the atom By Abhinav Anand

In 1900, J.J. Thomson discovered electrons in atoms. In 1886, E. Goldstein discovered positively charged canal rays, later identified as protons. In 1932, Chadwick discovered neutrons, neutral particles in atoms.
FINAL POWERPOINT PRESENTATION

1. Democritus was the first to propose that all matter is composed of tiny indivisible units called atoms. Later scientists like Dalton expanded on this idea.
2. Rutherford's gold foil experiment showed that atoms have a small, dense nucleus containing protons and neutrons, with electrons orbiting the nucleus. This overturned the plum pudding model.
3. Chadwick discovered the neutron in 1932, completing the standard picture of atomic structure with protons and neutrons in the nucleus and electrons in orbitals around it.
Discovery of atom

The Indians first attempted to discover atoms in the 6th century BCE, referring to the smallest particle as "anor". Several scientists like Lavoisier, Kekule, and Mendeleev attempted to organize the elements into a periodic table. Dmitri Mendeleev is generally credited with inventing the periodic table of chemical elements in 1869, arranging them into a table to illustrate recurring trends in their properties. The periodic table layout has been refined over time as new elements were discovered and new theoretical models explained chemical behavior. Safety tips are provided like not experimenting carelessly in the chemistry lab and maintaining clear concepts to build a strong foundation.
Chadwick

James Chadwick was a British physicist born in 1891 who is known for discovering the neutron. He received several honors for his work, including the Nobel Prize in Physics in 1935. Chadwick served in World War I and was a prisoner of war. He also participated in the Manhattan Project during World War II. Chadwick's model of the atom focused on neutrons, differing from Niels Bohr's model which depicted electrons in rings. Both models showed the structure of the atom but represented it differently.
James Chadwick

James Chadwick (1891-1974) was an English physicist who proved the existence of the neutron in 1932, for which he received the 1935 Nobel Prize in Physics. Chadwick was born in England and studied physics at Manchester University, earning his master's degree in 1913. He worked with Ernest Rutherford at the Cavendish Laboratory in Cambridge, where in 1932 he discovered the neutron through experiments bombarding beryllium with alpha particles. Chadwick's discovery of the neutron was crucial to enabling the development of nuclear weapons and nuclear power.
History of the Atom

The document outlines the development of atomic models from ancient Greece to the early 20th century. It describes the models of Democritus (400 BC), Dalton (1803), Thomson (1904), Rutherford (1911), and Bohr (1915). Democritus proposed that all matter is composed of indivisible atoms. Dalton established the theory that atoms of different elements have different atomic weights. Thomson discovered electrons within atoms. Rutherford provided evidence of a dense nucleus through gold foil experiments. Bohr determined electrons orbit in shells around the nucleus.
Stephen william hawking

Stephen Hawking was born in 1942 in Oxford, England. He studied physics and mathematics at Oxford University and later became the Lucasian Professor of Mathematics at Cambridge University. Hawking is considered one of the most notable theoretical physicists and is famous for his theories on black holes, theoretical cosmology, and quantum gravity. He authored the best-selling book "A Brief History of Time" and has sought to develop a complete understanding of the universe.
Contributions of eminent scientists

This document provides biographical information on several scientists:
- Isaac Newton was an English physicist and mathematician who discovered laws of motion and universal gravitation. He was born in 1643 and made seminal contributions to physics, mathematics, and optics.
- John Dalton was an English chemist who introduced atomic theory. He was born in 1766 and proposed atoms as indivisible particles and that elements combine in simple whole number ratios to form chemical compounds.
- Niels Bohr was a Danish physicist who made fundamental contributions to understanding atomic structure and quantum theory. He was born in 1885 and introduced the Bohr model of the atom in 1913.
- C.V. Raman was an Indian physicist known for his work on
Thomson Tube - em

1. The document describes an experiment to measure the charge-to-mass ratio of electrons using Thomson's cathode ray tube.
2. Two methods are used: 1) null deflection where electric and magnetic fields cancel each other out, and 2) deflection by a magnetic field alone where the radius of curvature is measured.
3. Equations are derived relating the experimental measurements to the charge-to-mass ratio. The results from both methods are within 2% of the accepted value, validating Thomson's original discovery of the electron.
The Atom

The document summarizes key concepts about atomic structure and isotopes. It explains that atoms are made up of protons, neutrons and electrons. The atomic number represents the number of protons, while the mass number is the total of protons and neutrons. Isotopes are atoms of the same element with different numbers of neutrons. The relative atomic mass takes into account the natural abundance of different isotopes in a sample.
More Related Content
What's hot
michael faraday

Michael Faraday was an English scientist who contributed significantly to the fields of electromagnetism and electrochemistry. Born into poverty in 1791, he received little formal education but became an assistant to scientist Humphry Davy. Through his own experimentation, Faraday discovered electromagnetic induction and invented the electric motor, laying the foundation for modern electricity. He also made important contributions to chemistry, discovering benzene and other aromatic compounds. Faraday introduced concepts of electromagnetic fields that became the basis of modern field theory in physics. He remained an influential scientist at the Royal Institution in London for over 50 years until his death in 1867.
James Chadwick

James Chadwick was a British physicist born in England. He studied under Ernest Rutherford and worked with him on experiments involving radioactive substances. This led Chadwick to continue Rutherford's idea that there were neutral particles in the nucleus of an atom. Through his own experiment in 1932, Chadwick was able to prove the existence of the neutron. He called these neutral particles "neutrons" and showed they have about the same mass as a proton but no electric charge. This discovery of the neutron was fundamental to modern physics and earned Chadwick the 1935 Nobel Prize in Physics.
Michael faraday powerpoint

Michael Faraday was born in 1791 in London to a poor family. He had little formal education but showed an interest in science through his work as a bookbinder. He became an assistant to scientist Humphry Davy at the Royal Institution, where he began his research into electricity and magnetism. Through experiments with coils and iron rings, he discovered the principles of electromagnetic induction and how changing magnetic fields can generate electric currents. His invention of the electric generator in 1831, which used a copper disk between magnet poles to produce a continuous electric current, allowed electricity to be efficiently produced and paved the way for widespread electrification.
Michael faraday

Michael Faraday was a British scientist who made many important contributions to the fields of
electromagnetism and electrochemistry in the 19th century. Through experiments such as discovering
electromagnetic induction and establishing the laws of electrolysis, Faraday laid the groundwork for modern
electric technology and helped scientists understand the relationship between electricity, magnetism, and
light. He published his findings in books and papers that communicated complex scientific concepts to
ordinary readers. Though born into poverty, Faraday rose to prominence through his work at the Royal
Institution of Great Britain, where he spent over 50 years conducting experiments and lectures.
Discovery of Electrons and Protons

This power point slides presents how the electrons and protons were discovered together with the personalities involved with this scientific breakthrough.
Michael Faraday

Michael Faraday was born in 1791 in England and had little formal education but became interested in science. He became an apprentice to scientist Humphry Davy, which launched his scientific career. Faraday made many important contributions including discovering benzene and two chlorine/carbon compounds. He invented the Bunsen burner and established the foundations of electromagnetism by producing the first electric motor and generator. Faraday published widely and popularized scientific terms and methods through lectures, making complex topics more accessible. He was honored with scientific medals and awards for his discoveries that still impact fields like chemistry and electricity today.
Michael faraday

Michael Faraday was an English scientist who made many important contributions to the fields of electromagnetism and electrochemistry. Though he received little formal education, through his own research he established the basis of the electromagnetic field and discovered the principles of electromagnetic induction and electrolysis. His inventions of electromagnetic devices formed the foundation of electric motor technology. He declined offers of knighthood and presidency of the Royal Society, preferring to remain "plain Mr. Faraday" throughout his life.
Faraday

Michael Faraday was a pioneering British scientist from a working class background who made seminal contributions to electromagnetism and electrochemistry through his patient, diligent experiments. His most influential inventions include the electric motor, generator and transformer. These breakthroughs helped establish the foundation of modern electricity and propelled society to new levels of technological development. Though honored with prestigious offers, Faraday remained humble and dedicated to scientific discovery throughout his life.
Ernest Rutherford

Ernest Rutherford conducted an experiment in 1911 where he fired positively charged alpha particles at a thin gold foil. Contrary to expectations, many particles were deflected or bounced back, showing that the atom's mass was concentrated in a small nucleus rather than evenly distributed as Thomson's plum pudding model predicted. This led Rutherford to propose the nuclear model of the atom, with electrons orbiting a dense central nucleus, revolutionizing the field of nuclear physics.
James chadwick

Chadwick discovered a new particle in the nucleus called the neutron through experiments bombarding various elements with alpha particles. The neutron was predicted by Majorana due to its lack of an electrical charge, allowing it to penetrate nuclei without overcoming electromagnetic repulsion like protons. Using Rutherford's zinc shield technique, Chadwick determined neutrons could enter even the heaviest element nuclei, helping establish the neutron as a key part of the nuclear model and defining the atom's mass.
MICHAEL FARADAY PPT..

life style of great scientist Michael Faraday .....!
Michael Faraday, who came from a very poor family, became one of the greatest scientists in history. His achievement was remarkable in a time when science was the preserve of people born into privileged families. The unit of electrical capacitance is named the farad in his honor, with the symbol F.
The faraday is a dimensionless unit of electric charge quantity, equal to approximately 6.02 x 10 23 electric charge carriers. This is equivalent to one mole , also known as Avogadro's constant .
Education and Early Life
Michael Faraday was born on September 22, 1791 in London, England, UK. He was the third child of James and Margaret Faraday. His father was a blacksmith who had poor health. Before marriage, his mother had been a servant. The family lived in a degree of poverty.
Michael Faraday attended a local school until he was 13, where he received a basic education. To earn money for the family he started working as a delivery boy for a bookshop. He worked hard and impressed his employer. After a year, he was promoted to become an apprentice bookbinder
Michael Faraday’s Scientific Achievements and Discoveries:
It would be easy fill a book with details of all of Faraday’s discoveries – in both chemistry and physics. It is not an accident that Albert Einstein used to keep photos of three scientists in his office: Isaac Newton, James Clerk Maxwell and Michael Faraday.
Funnily enough, although in Faraday’s lifetime people had started to use the word physicist, Faraday disliked the word and always described himself as a philosopher. 1821: Discovery of Electromagnetic Rotation
This is a glimpse of what would eventually develop into the electric motor, based on Hans Christian Oersted’s discovery that a wire carrying electric current has magnetic properties.
1823: Gas Liquefaction and Refrigeration
In 1802 John Dalton had stated his belief that all gases could be liquified by the use of low temperatures and/or high pressures. Faraday provided hard evidence for Dalton’s belief by applying pressure to liquefy chlorine gas and ammonia gas for the first time.
1825: Discovery of Benzene
Historically, benzene is one of the most important substances in chemistry, both in a practical sense – i.e. making new materials; and in a theoretical sense – i.e. understanding chemical bonding. Michael Faraday discovered benzene in the oily residue left behind from producing gas for lighting in London.
1831: Discovery of Electromagnetic Induction
Faraday discovered that a varying magnetic field causes electricity to flow in an electric circuit.
1834: Faraday’s Laws of Electrolysis
This is the science of understanding what happens at the interface of an electrode with an ionic substance. Electrochemistry is the science that has produced the Li ion batteries and metal hydride batteries capable of powering modern mobile technology. Faraday’s laws are vital to our understanding of electrode reactions.
discovery of proton 

Eugen Goldstein was a German physicist born in 1850 who made early investigations of discharge tubes and discovered anode rays. He is sometimes credited with the discovery of the proton. In the 1870s, Goldstein undertook his own investigations of discharge tubes and named the light emissions studied by others cathode rays. Goldstein conducted experiments with canal rays that contributed to the discovery of the proton.
Structure of the atom By Abhinav Anand

In 1900, J.J. Thomson discovered electrons in atoms. In 1886, E. Goldstein discovered positively charged canal rays, later identified as protons. In 1932, Chadwick discovered neutrons, neutral particles in atoms.
FINAL POWERPOINT PRESENTATION

1. Democritus was the first to propose that all matter is composed of tiny indivisible units called atoms. Later scientists like Dalton expanded on this idea.
2. Rutherford's gold foil experiment showed that atoms have a small, dense nucleus containing protons and neutrons, with electrons orbiting the nucleus. This overturned the plum pudding model.
3. Chadwick discovered the neutron in 1932, completing the standard picture of atomic structure with protons and neutrons in the nucleus and electrons in orbitals around it.
Discovery of atom

The Indians first attempted to discover atoms in the 6th century BCE, referring to the smallest particle as "anor". Several scientists like Lavoisier, Kekule, and Mendeleev attempted to organize the elements into a periodic table. Dmitri Mendeleev is generally credited with inventing the periodic table of chemical elements in 1869, arranging them into a table to illustrate recurring trends in their properties. The periodic table layout has been refined over time as new elements were discovered and new theoretical models explained chemical behavior. Safety tips are provided like not experimenting carelessly in the chemistry lab and maintaining clear concepts to build a strong foundation.
Chadwick

James Chadwick was a British physicist born in 1891 who is known for discovering the neutron. He received several honors for his work, including the Nobel Prize in Physics in 1935. Chadwick served in World War I and was a prisoner of war. He also participated in the Manhattan Project during World War II. Chadwick's model of the atom focused on neutrons, differing from Niels Bohr's model which depicted electrons in rings. Both models showed the structure of the atom but represented it differently.
James Chadwick

James Chadwick (1891-1974) was an English physicist who proved the existence of the neutron in 1932, for which he received the 1935 Nobel Prize in Physics. Chadwick was born in England and studied physics at Manchester University, earning his master's degree in 1913. He worked with Ernest Rutherford at the Cavendish Laboratory in Cambridge, where in 1932 he discovered the neutron through experiments bombarding beryllium with alpha particles. Chadwick's discovery of the neutron was crucial to enabling the development of nuclear weapons and nuclear power.
History of the Atom

The document outlines the development of atomic models from ancient Greece to the early 20th century. It describes the models of Democritus (400 BC), Dalton (1803), Thomson (1904), Rutherford (1911), and Bohr (1915). Democritus proposed that all matter is composed of indivisible atoms. Dalton established the theory that atoms of different elements have different atomic weights. Thomson discovered electrons within atoms. Rutherford provided evidence of a dense nucleus through gold foil experiments. Bohr determined electrons orbit in shells around the nucleus.
Stephen william hawking

Stephen Hawking was born in 1942 in Oxford, England. He studied physics and mathematics at Oxford University and later became the Lucasian Professor of Mathematics at Cambridge University. Hawking is considered one of the most notable theoretical physicists and is famous for his theories on black holes, theoretical cosmology, and quantum gravity. He authored the best-selling book "A Brief History of Time" and has sought to develop a complete understanding of the universe.
Contributions of eminent scientists

This document provides biographical information on several scientists:
- Isaac Newton was an English physicist and mathematician who discovered laws of motion and universal gravitation. He was born in 1643 and made seminal contributions to physics, mathematics, and optics.
- John Dalton was an English chemist who introduced atomic theory. He was born in 1766 and proposed atoms as indivisible particles and that elements combine in simple whole number ratios to form chemical compounds.
- Niels Bohr was a Danish physicist who made fundamental contributions to understanding atomic structure and quantum theory. He was born in 1885 and introduced the Bohr model of the atom in 1913.
- C.V. Raman was an Indian physicist known for his work on
What's hot (20)
Viewers also liked
Thomson Tube - em

1. The document describes an experiment to measure the charge-to-mass ratio of electrons using Thomson's cathode ray tube.
2. Two methods are used: 1) null deflection where electric and magnetic fields cancel each other out, and 2) deflection by a magnetic field alone where the radius of curvature is measured.
3. Equations are derived relating the experimental measurements to the charge-to-mass ratio. The results from both methods are within 2% of the accepted value, validating Thomson's original discovery of the electron.
The Atom

The document summarizes key concepts about atomic structure and isotopes. It explains that atoms are made up of protons, neutrons and electrons. The atomic number represents the number of protons, while the mass number is the total of protons and neutrons. Isotopes are atoms of the same element with different numbers of neutrons. The relative atomic mass takes into account the natural abundance of different isotopes in a sample.
(Classen) Electron/ mass ratio lab report

The document summarizes an experiment to determine the charge to mass ratio of an electron (e/m) using a Helmholtz coil apparatus. It provides background on the theory and setup of the experiment. Data was collected on the magnetic field and electron beam radius needed to hit targets of different distances. The average calculated e/m ratios were 2.24065 × 1011 c/kg and 2.01474 × 1011 c/kg for magnetic fields lined with and against the Earth's field, respectively. While these differed from the accepted value, the author notes factors like measurement precision that could have contributed to the error.
JJ Thomson experiment (1897)

J.J. Thomson conducted a series of experiments using cathode ray tubes that led to the discovery of the electron. In his first experiment, he observed that an electric current caused a beam to move from the negative cathode to the positive anode. His second experiment showed this beam was composed of invisible rays emitted from the negative plate. His third experiment applied an electric field and showed the particles composing the beam were negatively charged. His fourth experiment under changed conditions still produced the same results, allowing him to infer that all substances contain negatively charged particles, discovering the electron.
Chapter 2 structure of atom class 11

1) Experiments with cathode ray tubes led to the discovery of the electron as a negatively charged fundamental particle.
2) Further experiments showed that atoms are mostly empty space and contain a small, dense nucleus made up of protons and neutrons, around which electrons orbit.
3) The photoelectric effect showed that light behaves as a particle (photon) rather than just a wave, transferring its energy in discrete quantized amounts to electrons and ejecting them from metal surfaces.
Mass spectrometry

The document provides information about mass spectrometry including:
- Mass spectrometry is a powerful analytical technique that uses instruments called mass spectrometers to identify molecules by breaking them into ionized fragments and measuring their mass-to-charge ratios.
- The basic components of a mass spectrometer are the sample inlet, ionization source, mass analyzer, and ion detector. Common ionization sources are electrospray ionization, matrix-assisted laser desorption/ionization, and electron ionization. Common mass analyzers are quadrupoles, ion traps, and time-of-flight.
- Mass spectrometry has a variety of applications and has undergone significant technological developments since its invention in the early 20th
Mass spectrometry

Mass spectrometry is a technique that converts a sample to gas-phase ions which are then separated by mass and charge. It involves ionization of the sample using electron bombardment or other methods, mass analysis using magnetic or electric fields to separate ions, and detection of ion abundances. Mass spectrometry can be used to determine molecular masses and obtain structural information through fragmentation patterns.
Viewers also liked (7)
Similar to 1 ch3 student to complete their_notes_12_13_
Structure of Atom Class 9 CBSE PPT-1.pptx

This document discusses several historical models of the atom. It begins with Dalton's atomic theory from the 1800s, then covers J.J. Thomson's "plum pudding" model from the 1890s, in which electrons were embedded in a uniform sphere of positive charge. In the early 1900s, Rutherford's gold foil experiment showed that the positive charge and mass of atoms are concentrated in a small, dense nucleus. This led to Rutherford's nuclear model of the atom. However, this model was unable to explain the stability of atoms. Niels Bohr then proposed discrete, quantized electron orbits around the nucleus in 1913 to explain atomic stability.
Unit 4. the atom

Rutherford's gold foil experiment in 1909 led to the discovery that atoms have a small, dense nucleus containing positive charge. This contradicted the prevailing Thomson "plum pudding" model, which depicted atoms as uniformly positive with electrons mixed in. Through deflecting alpha particles, Rutherford deduced atoms are mostly empty space with a tiny, massive nucleus at their center. He thus proposed the planetary model of the atom, with electrons orbiting the nucleus similarly to planets around the Sun. However, this model failed to explain atomic stability, until Niels Bohr introduced quantum theory in 1913, proposing electrons occupy discrete energy levels and can jump between them by absorbing or emitting photons. This evolution of atomic theory progressed
iesc104.pdf

The document discusses the structure of atoms and the discovery of subatomic particles. It explains that:
1) Early experiments showed atoms were divisible and contained electrons and protons.
2) Rutherford's gold foil experiment showed atoms had a small, dense nucleus containing most of the mass.
3) Models were developed to explain the atom's structure, including Thomson's "plum pudding" model and Rutherford's nuclear model.
4) Later, the Bohr model incorporated allowed electron orbits, and neutrons were discovered in atomic nuclei.
athe structure of atom class -9th.pdf

The document discusses the structure of the atom. It explains the key discoveries and models that helped reveal the internal structure of atoms, including Thomson's plum pudding model, Rutherford's nuclear model based on his gold foil experiment, and Bohr's model incorporating allowed electron orbits. It describes the subatomic particles - electrons, protons, and neutrons - and how they are arranged in the nucleus and electron shells. The document also discusses concepts like atomic number, mass number, isotopes, valency, and electronic configuration.
Atomic theory notes

This document provides an overview of the development of atomic theory from ancient Greek philosophers to modern atomic structure. It summarizes key contributors and discoveries:
- Democritus proposed atoms as indivisible particles (5th century BC). John Dalton's atomic theory (1803) stated all matter is made of atoms that cannot be created, destroyed, or divided.
- J.J. Thomson's discovery of the electron (1897) showed atoms can be divided. Ernest Rutherford's gold foil experiment (1909) demonstrated atoms are mostly empty space with a dense nucleus.
- Niels Bohr's model (1913) showed electrons orbiting the nucleus in defined energy levels. Modern atomic theory describes electrons in
History of the atom

The document provides a history of discoveries related to the atom from ancient Greek philosophers to modern quantum mechanics. It describes key contributors such as Democritus proposing atoms, Dalton establishing atomic theory, Rutherford discovering the nucleus, Bohr introducing quantum mechanics, and Heisenberg establishing the uncertainty principle. The development of atomic models progressed from simple spheres to planetary structures to quantum mechanical probability distributions.
Avi

The document discusses the structure of atoms. It explains that atoms are made up of subatomic particles like electrons, protons, and neutrons. J.J. Thomson discovered the electron, while E. Goldstein discovered the positively charged particle, which was later named the proton. Ernest Rutherford's alpha particle scattering experiment provided evidence that the mass and positive charge of an atom are concentrated in a small, dense nucleus at the center. Niels Bohr later proposed that electrons orbit the nucleus in well-defined energy levels or shells. In 1932, James Chadwick discovered the neutron, which has no charge and a mass similar to a proton. The structure of atoms is defined by the number of protons, which determines the element, and
Atomic structure

The document discusses the history of the development of atomic structure models from Thomson's plum pudding model to Rutherford's nuclear model. Key events include J.J. Thomson's discovery of the electron, Millikan's oil drop experiment determining the charge of an electron, discovery of the proton through canal ray experiments, Rutherford's alpha particle scattering experiment revealing the dense nucleus at the center of the atom, and Rutherford proposing the nuclear model of the atom. The nuclear model represented a major breakthrough but did not fully explain electron stability.
MODELS OF THE atom......................

Three models contributed to developing the modern understanding of the atom:
1. Dalton's model proposed that atoms of different elements have different properties and that atoms cannot be subdivided further.
2. Thomson's "plum pudding" model viewed the atom as a sphere of positive charge with electrons embedded inside.
3. Rutherford's gold foil experiment revealed that the atom consists of a small, dense nucleus surrounded by electrons, overturning Thomson's model and leading to further developments.
Atomic theory gr 9

1. Dalton proposed the first atomic theory in 1808, which stated that all matter is made of atoms that cannot be created, destroyed, or subdivided during chemical reactions.
2. In the early 1900s, scientists like Thomson, Rutherford, and Faraday expanded on Dalton's theory by discovering that atoms contain electrons and identifying the nucleus as the center of the atom that contains protons and neutrons.
3. Rutherford further amended atomic theory in 1911 by concluding that the nucleus contains nearly all the mass of an atom and that electrons orbit the nucleus, leaving mostly empty space inside atoms.
Atomic theory gr 9 -may 24

1. Dalton proposed the first atomic theory in 1808, which stated that all matter is made of atoms that cannot be created, destroyed, or subdivided during chemical reactions.
2. In the early 1900s, scientists like Thomson, Rutherford, and Faraday contributed new discoveries about the structure of atoms, such as electrons, positive and negative charges, and the nucleus.
3. Rutherford's 1911 gold foil experiment provided evidence that atoms have a small, dense nucleus containing protons and neutrons, with electrons orbiting the outside, and largely empty space in between. This led to revisions of atomic theory.
Atomic Structure ememememememmememeeemmemememememem

This document discusses the early discoveries and models of the atom. It describes J.J. Thomson's discovery of the electron using cathode ray tubes. Robert Millikan then measured the charge and mass of individual electrons using an oil drop experiment. Ernest Rutherford used gold foil experiments to determine that the atom has a small, dense, positively charged nucleus at its center. Niels Bohr later incorporated Rutherford's nuclear model and proposed that electrons orbit the nucleus in fixed shells.
Atomic Structure ememememememmememeeemmemememememem

This document discusses the early discoveries and models of the atom. It describes J.J. Thomson's discovery of the electron using cathode ray tubes. Robert Millikan then measured the charge and mass of individual electrons using an oil drop experiment. Ernest Rutherford used gold foil experiments to determine that the atom has a small, dense, positively charged nucleus at its center. Niels Bohr later incorporated Rutherford's nuclear model and proposed that electrons orbit the nucleus in fixed shells.
Thomson atom model

J.J. Thomson was a British physicist born in 1856 who made several important discoveries about the structure of atoms. He discovered the electron in 1897 and proposed the "plum pudding" model of the atom, which depicted the atom as a ball of positive charge with electrons embedded inside. In 1904, Thomson discovered the atomic nucleus at the center of the atom. His work established that atoms can be divided into smaller parts.
Historical Development of the Atom model of an atom.ppt

The document traces the history of atomic models from ancient Greek philosophers to modern quantum theory. It begins with Democritus' idea in 460 BC that all matter is made of indivisible atoms. In the early 1800s, Dalton proposed atoms of different elements have unique properties and combine in fixed ratios. J.J. Thomson discovered atoms contain negatively charged electrons. Rutherford's gold foil experiment in 1911 revealed the dense, positively charged nucleus. Later, Chadwick found neutrons in the nucleus and Bohr proposed electron orbits. Quantum theory with Schrodinger and Heisenberg established atoms as probability clouds rather than definite particles.
Lesson 4 Not Indivisible (The Structure of the Atom)

Learning Competencies
At the end of the lesson, you will have to:
1. point out the main ideas in the discovery of the structure of the atom and its subatomic particles
2. cite the contributions of J.J. Thomson, Ernest Rutherford, Henry Moseley, and Niels Bohr to the understanding of the structure of the atom
3. describe the nuclear model of the atom and the location of its major components (protons, neutrons, and electrons)
Model of the_atom

The document describes the development of the atomic model over time. John Dalton proposed the first atomic theory in the early 1800s, stating that elements are made of atoms and atoms of different elements are distinct. Later, scientists like Crookes, Thomson, and Rutherford provided evidence through experiments that led to refinements of the model. Thomson discovered the electron and proposed that atoms contain positive charge distributed throughout, but Rutherford's gold foil experiment showed that positive charge is concentrated in a small nucleus. Chadwick later discovered the neutron in the nucleus. [/SUMMARY]
5.2 Newer models of the Atom

1) The document summarizes the major historical atomic models from Dalton to Bohr, including Thomson's "plum pudding" model where electrons were distributed evenly in the positively charged atom, Rutherford's gold foil experiment which showed that the atom is mostly empty space with a small, dense nucleus, and Bohr's planetary model where electrons orbit the nucleus in specific paths.
2) It introduces JJ Thomson's discovery of electrons as negatively charged particles within atoms through cathode ray experiments and his development of the plum pudding model.
3) It also mentions James Chadwick's discovery of the neutron, which helped explain the mass of atoms.
stucture of atom 

structure of atom study material
please consider to follow it gives me education only 1 % of viewers are following its free !
Similar to 1 ch3 student to complete their_notes_12_13_ (20)
Atomic Structure ememememememmememeeemmemememememem

Atomic Structure ememememememmememeeemmemememememem
Atomic Structure ememememememmememeeemmemememememem

Atomic Structure ememememememmememeeemmemememememem
Historical Development of the Atom model of an atom.ppt

Historical Development of the Atom model of an atom.ppt
Lesson 4 Not Indivisible (The Structure of the Atom)

Lesson 4 Not Indivisible (The Structure of the Atom)
More from Chris Hitchens
Chapter 3 do it yourself admissions

Getting into college is competitive, so taking steps to stand out from other applicants can help your chances of admission. Some things students can do themselves to boost their applications include writing compelling essays that highlight their unique experiences and qualities, asking teachers for recommendation letters that emphasize their strengths, and researching schools to find the best fit for their interests and abilities. Overall, focusing the application on fit and putting your best foot forward are important for students aiming to gain admission to their top college choices.
Chapter 2 rankings up and down

The document discusses the benefits of meditation for reducing stress and anxiety. Regular meditation practice can help calm the mind and body by lowering heart rate and blood pressure. Studies have shown that meditating for just 10-20 minutes per day can have significant positive impacts on both mental and physical health.
Chapter 1 The Platinum Package

This document appears to contain numerical codes or identifiers. It includes 6 numbers or codes with slashes or dashes between some of the values. The document provides identifying or classification information but does not contain much contextual meaning on its own.
Flipping the classroom final edition inservice

This document discusses flipping the classroom model of education. It begins with some introductory slides on flipping the classroom and outlines what it is and is not. It then discusses two different philosophies for flipping - fostering curiosity and mastery learning. Several examples are provided of how to "flip" the environment and sequence of lessons using Bloom's taxonomy. The remainder discusses tools and resources for flipping including hosting videos, screencasting, and curating existing video content as well as blogs and examples to consult.
Ch 5 Notes Part 1

The periodic table has evolved over time as more elements were discovered. Early scientists like Dobereiner noticed patterns among triads of elements and proposed the Law of Triads. Mendeleev arranged the known elements by atomic mass and properties into the first recognizable periodic table, which left gaps that he predicted would be filled by undiscovered elements like gallium and germanium. Moseley discovered the relationship between atomic number and x-ray spectra, establishing atomic number as fundamental. Later discoveries like the noble gases contributed to the modern form of the periodic table.
10 chapt3 text part1

The document discusses three scientific laws: the Law of Conservation of Mass states that the mass of products in a chemical reaction equals the mass of reactants; the Law of Definite Proportions states that elements combine in fixed ratios by mass in compounds; the Law of Multiple Proportions states that when the same two elements react together to form more than one compound, the ratios of the mass of one element that combines with a fixed mass of the other element will be in the ratio of small whole numbers.
Modern Chemistry Sec 2.2

The document discusses the benefits of exercise for mental health. Regular physical activity can help reduce anxiety and depression and improve mood and cognitive functioning. Exercise boosts blood flow, releases endorphins, and promotes changes in the brain which help enhance one's emotional well-being and mental clarity.
Sect2 1 The Scientific Method

This short document appears to mark the end of a chapter section in another work. It provides no other context or content to summarize.
Graduation project oral presentation rubric

This document contains a rubric for evaluating oral presentations of a graduation project. It evaluates students on four key areas: project content, presentation content, organization, and oral presentation skills. It provides scoring criteria for each area, with points allocated for thoroughly and partially addressing criteria. To pass, students must score 30/50 or higher. Areas evaluated include addressing career exploration activities, demonstrating an understanding of the career exploration process, organization of the presentation, language usage, gestures, eye contact, speech clarity, pace, professional appearance, and presentation length.
Reflection rubric for evaluation day

This rubric evaluates reflection papers for a graduation project on career research and exploration. Students must write a reflection paper on one of the following activities: a career fair, goal setting, or exploring two careers through interest surveys, employer interviews, job shadows, or electronic career searches. The rubric assesses the papers on focus, content, organization, and conventions. Exceptional papers will clearly state the purpose, include a thorough reflective analysis with summary and conclusions. Proficient papers imply the purpose and include a reflective analysis with summary and conclusions. Papers that receive no credit do not state the purpose and lack development and reflection.
Embed google calendar1

To embed a Google Calendar on a website or blog, copy the iframe code from the Calendar settings page of the calendar you want to embed. Paste this code into the HTML of your website or blog post and publish. The embedded calendar will then be visible to anyone who views the site, as well as those you have shared the calendar with in Google Calendar.
Ch4 lesson 5 electron notation configuration_theory

The document provides an analogy comparing the positions of electrons in atoms to the positions of people in a bomb shelter. It explains that electrons have specific coordinates defined by their energy level, shape, and number. The first few elements (hydrogen, helium, lithium, beryllium, boron) are used as examples to demonstrate how to write the electronic configuration (coordinates) of each element based on the number of electrons. The key points are that electrons fill the lowest available energy levels and shapes according to the aufbau principle and Pauli exclusion principle.
Ch4 lesson 4 sect 2 waves

This document discusses an invisible boy who can become invisible when no one is looking at him, and draws an analogy between this and the behavior of electrons. Electrons act as mathematical functions and their trajectory can be predicted but their precise location is unknown, similar to how the invisible boy can be located when observed but becomes invisible otherwise. The document relates the invisible boy's powers to the observable properties of electrons.
Ch4 lesson 5 calculations ii e_h_v

This document contains a lesson on calculating energy using Planck's constant and frequency. It introduces the energy equation E=hv, where E is energy, h is Planck's constant, and v is frequency. It then provides two example problems - one calculating the energy of a photon with a given frequency, and the other calculating the frequency of a wave with a given energy. The document guides the reader through solving each example problem step-by-step.
College planning values interpretation1

This document provides suggestions for college selection based on different values that may be important to students in the college planning process. It discusses 10 different values that could be priorities and suggests colleges and factors to consider for each value. For example, it recommends primarily undergraduate institutions and low student-faculty ratios for students for whom academics is the top priority, and colleges with opportunities for recognition for students who value being known for their success in an area of interest. The document aims to help students align their college selection process with their important personal values.
Self knowledge interpretation1 1

The document provides interpretation and advice for each response on a self-knowledge questionnaire regarding preparation and skills for college. It analyzes responses of 3, 4, or 5 which indicate areas for improvement. For academic abilities, it advises working to strengthen skills and considering colleges that can provide help. For study skills and time management, it recommends seeking help to improve organization. For motivation, it suggests reflecting on what type of college environment would be most motivating. For decision-making, it gives tips for gathering information from multiple sources to make the best choice.
More from Chris Hitchens (20)
Ch4 lesson 5 electron notation configuration_theory

Ch4 lesson 5 electron notation configuration_theory
1 ch3 student to complete their_notes_12_13_
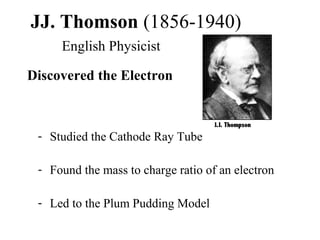
- 1. JJ. Thomson (1856-1940) English Physicist Discovered the Electron - Studied the Cathode Ray Tube - Found the mass to charge ratio of an electron - Led to the Plum Pudding Model
- 2. The Design of the Cathode Ray Tube
- 3. So! Thompson has discovered the electron, and it must live inside atoms. It is much less massive than the the atom itself, so perhaps we have little electrons stuffed into the ‘rest’ of the atom like raisins in the oatmeal, or: Plum Pudding...
- 4. Robert Milliken (1868-1953) American Physicist Performed the Oil Drop Experiment – Measured minimum electric charge that could be carried by a particle (1905) – This was the charge of the electron – Enabled him to find the mass of the electron
- 5. Millikan’s Oil Drop Experiment Used To Determine the Charge of an Electron
- 6. Ernest Rutherford (1871-1953) • New Zealand born physicist • Worked with nuclear radiation • Performed the Gold Foil Experiment • Discovered the nucleus (1910) • Discovered the proton (1918)
- 7. The Gold Foil Experiment Demonstrated that the atom contains a nucleus which is: a. Very tiny – most of the particles missed b. Positively charged – some were deflected c. Very dense – some bounced off
- 8. James Chadwick (1891-1974) • Discovered the neutron in 1932 • In college he stood in the wrong line. He wanted to be a mathematician, but was in the line for physics and was too embarrassed to switch. • Instead he became a famous physicist.
- 9. End! The story’s not over, but we’ll pick it up in chapter 4.
