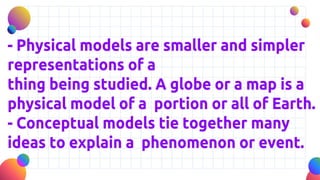
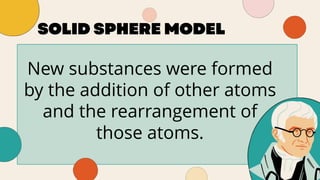
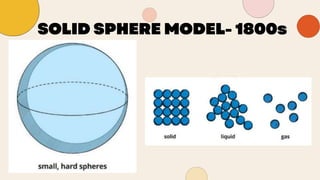
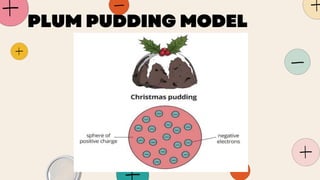
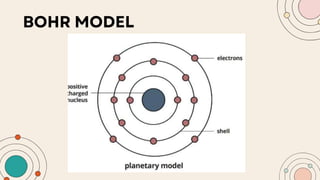
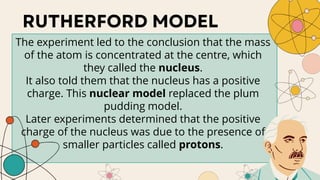
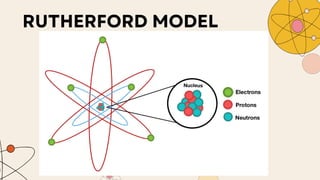
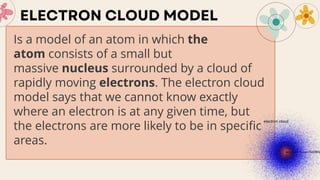
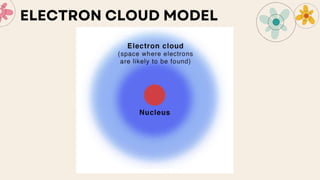
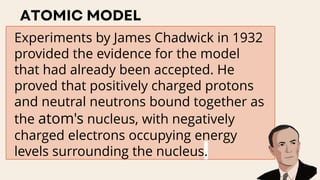
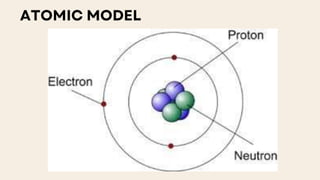
John Dalton proposed that matter is made of indivisible atoms, leading to the development of atomic models over time. J.J. Thomson introduced the plum pudding model, which was replaced by Rutherford's nuclear model after experiments demonstrated that atoms have a dense, positively charged nucleus. Later, the electron cloud model emerged, with contributions from Niels Bohr and James Chadwick, detailing electrons' behavior around the nucleus formed by protons and neutrons.