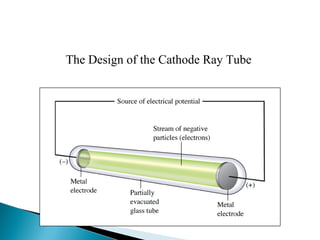
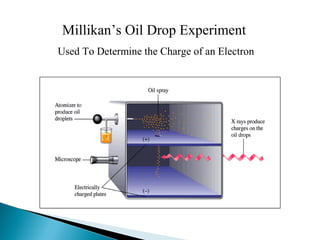
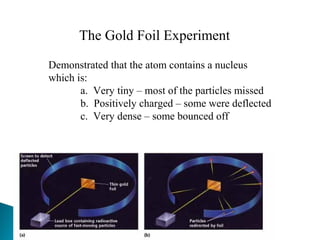
J.J. Thomson discovered the electron through experiments with cathode ray tubes. He found the mass to charge ratio of electrons and proposed the plum pudding model of the atom, where electrons are embedded in a positively charged material. Robert Millikan performed the oil drop experiment, which measured the minimum electric charge and enabled finding the mass of the electron. Ernest Rutherford conducted the gold foil experiment, which demonstrated that atoms have a small, dense, positively charged nucleus.